
rexresearch
rexresearch
Liang-Shih FAN
Coal-Direct Chemical Looping
http://hardware.slashdot.org/story/13/02/21/2336200/new-process-takes-energy-from-coal-without-burning-it
New Process Takes Energy From Coal Without Burning It"Ohio State students have come up with a scaled-down version of a power plant combustion system with a unique experimental design--one that chemically converts coal to heat while capturing 99 percent of the carbon dioxide produced in the reaction. Typical coal-fired power plants burn coal to heat water to make steam, which turns the turbines that produce electricity. In chemical looping, the coal isn't burned with fire, but instead chemically combusted in a sealed chamber so that it doesn't pollute the air. This new technology, called coal-direct chemical looping, was pioneered by Liang-Shih Fan, professor of chemical and biomolecular engineering and director of Ohio State's Clean Coal Research Laboratory."
http://researchnews.osu.edu/archive/looping203.htm
Ohio State University Research and Innovation Communications
New Coal Technology Harnesses Energy Without Burning, Nears Pilot-Scale Development
by Pam Gorder
COLUMBUS, Ohio—A new form of clean coal technology reached an important milestone recently, with the successful operation of a research-scale combustion system at Ohio State University. The technology is now ready for testing at a larger scale.
For 203 continuous hours, the Ohio State combustion unit produced heat from coal while capturing 99 percent of the carbon dioxide produced in the reaction.
Liang-Shih Fan, professor of chemical and biomolecular engineering and director of Ohio State’s Clean Coal Research Laboratory, pioneered the technology called Coal-Direct Chemical Looping (CDCL), which chemically harnesses coal’s energy and efficiently contains the carbon dioxide produced before it can be released into the atmosphere.
“In the simplest sense, combustion is a chemical reaction that consumes oxygen and produces heat,” Fan said. “Unfortunately, it also produces carbon dioxide, which is difficult to capture and bad for the environment. So we found a way to release the heat without burning. We carefully control the chemical reaction so that the coal never burns—it is consumed chemically, and the carbon dioxide is entirely contained inside the reactor.”
Dawei Wang, a research associate and one of the group's team leaders, described the technology’s potential benefits. "The commercial-scale CDCL plant could really promote our energy independence. Not only can we use America's natural resources such as Ohio coal, but we can keep our air clean and spur the economy with jobs," he said.
“We carefully control the chemical reaction so that the coal never burns—it is consumed chemically, and the carbon dioxide is entirely contained inside the reactor.”
Though other laboratories around the world are trying to develop similar technology to directly convert coal to electricity, Fan’s lab is unique in the way it processes fossil fuels. The Ohio State group typically studies coal in the two forms that are already commonly available to the power industry: crushed coal “feedstock,” and coal-derived syngas.
The latter fuel has been successfully studied in a second sub-pilot research-scale unit, through a similar process called Syngas Chemical Looping (SCL). Both units are located in a building on Ohio State’s Columbus campus, and each is contained in a 25-foot-high insulated metal cylinder that resembles a very tall home water heater tank.
No other lab has continuously operated a coal-direct chemical looping unit as long as the Ohio State lab did last September. But as doctoral student Elena Chung explained, the experiment could have continued.
“We voluntarily chose to stop the unit. We actually could have run longer, but honestly, it was a mutual decision by Dr. Fan and the students. It was a long and tiring week where we all shared shifts,” she said.
Fan agreed that the nine-day experiment was a success. “In the two years we’ve been running the sub-pilot plants, our CDCL and SCL units have achieved a combined 830 operating hours, which clearly demonstrates the reliability and operability of our design,” he said.
At any one time, the units each produce about 25 thermal kilowatts—that is, thermal energy, which in a full-scale power plant would be used to heat water and turn the steam-powered turbines that create electricity.
The researchers are about to take their technology to the next level: a larger-scale pilot plant is under construction at the U.S. Department of Energy’s National Carbon Capture Center in Wilsonville, AL. Set to begin operations in late 2013, that plant will produce 250 thermal kilowatts using syngas.
The key to the technology is the use of tiny metal beads to carry oxygen to the fuel to spur the chemical reaction. For CDCL, the fuel is coal that’s been ground into a powder, and the metal beads are made of iron oxide composites. The coal particles are about 100 micrometers across—about the diameter of a human hair—and the iron beads are larger, about 1.5-2 millimeters across. Chung likened the two different sizes to talcum powder and ice cream sprinkles, though the mix is not nearly so colorful.
The coal and iron oxide are heated to high temperatures, where the materials react with each other. Carbon from the coal binds with the oxygen from the iron oxide and creates carbon dioxide, which rises into a chamber where it is captured. Hot iron and coal ash are left behind. Because the iron beads are so much bigger than the coal ash, they are easily separated out of the ash, and delivered to a chamber where the heat energy would normally be harnessed for electricity. The coal ash is removed from the system.
The carbon dioxide is separated and can be recycled or sequestered for storage. The iron beads are exposed to air inside the reactor, so that they become re-oxidized be used again. The beads can be re-used almost indefinitely, or recycled.
Since the process captures nearly all the carbon dioxide, it exceeds the goals that DOE has set for developing clean energy. New technologies that use fossil fuels should not raise the cost of electricity more than 35 percent, while still capturing more than 90 percent of the resulting carbon dioxide. Based on the current tests with the research-scale plants, Fan and his team believe that they can meet or exceed that requirement.
The DOE funded this research, and collaborating companies include Babcock & Wilcox Power Generation Group, Inc.; CONSOL Energy, Inc.; and Clear Skies Consulting, LLC.
Contacts: L.-S. Fan, (614) 688-3262; Fan.1@osu.edu
Elena Chung, (614) 247-2787; Chung.461@osu.edu
Liang-Shih Fan
[0002] The present invention is generally directed to systems and methods of converting fuel, and is generally directed to oxidation-reduction reactor systems used in fuel conversion.
[0003] There is a constant need for clean and efficient energy generation systems. Most of the commercial processes that generate energy carriers such as steam, hydrogen, synthesis gas (syngas), liquid fuels and/or electricity are based on fossil fuels. Furthermore, the dependence on fossil fuels is expected to continue in the foreseeable future due to the much lower costs compared to renewable sources. Currently, the conversion of carbonaceous fuels such as coal, natural gas, petroleum coke is usually conducted through a combustion or reforming process. However, combustion of carbonaceous fuels, especially coal, is a carbon intensive process that emits large quantities of carbon dioxide to the environment. Sulfur and nitrogen compounds are also generated in this process due to the complex content in coal.
[0004] Chemical reactions between metal oxides and carbonaceous fuels, on the other hand, may provide a better way to recover the energy stored in the fuels. Several processes are based on the reaction of metal oxide particles with carbonaceous fuels to produce useful energy carriers. For example, Ishida et al. U.S. Pat. No. 5,447,024 describes processes wherein nickel oxide particles are used to convert natural gas through a chemical looping process into heat, which may be used in a turbine. However, recyclability of pure metal oxides is poor and constitutes an impediment for its use in commercial and industrial processes. Moreover, this technology has limited applicability, because it can only convert natural gas, which is more costly than other fossil fuels. Another well known process is a steam-iron process, wherein coal derived producer gas is reacted with iron oxide parlicles in a fiuidized bed reactor to be later regenerated with steam to produce hydrogen gas. This process however suffers from poor gas conversion -0-
[0005] rates due to improper contact between reacting solids and gases, and is incapable of producing a hydrogen rich stream.
[0006] As demands increase for cleaner and more efficient systems of converting fuel, the need arises for improved systems, and system components therein, which will convert fuel effectively, while reducing pollutants.
[0007] In one embodiment of the present invention, a system for converting fuel is provided. The system comprises a first reactor comprising a plurality of ceramic composite particles, wherein the ceramic composite particles comprise at least one metal oxide disposed on a support. The first reactor is configured to reduce at least one metal oxide with a fuel to produce a reduced metal or a reduced metal oxide. The system also comprises a second reactor configured to oxidize the reduced metal or reduced metal oxide to produce a metal oxide intermediate, and a third reactor configured to regenerate at least one metal oxide by oxidizing the metal oxide intermediate.
[0008] In another embodiment of the present invention, a method of converting fuel to hydrogen, CO, or syngas is provided. The method comprises the steps of: reducing a metal oxide in a reduction reaction between a fuel and a metal oxide to a reduced metal or a reduced metal oxide; oxidizing the reduced metal or reduced metal oxide with an oxidant to a metal oxide intermediate, while also producing hydrogen, CO, or syngas; and regenerating the at least one metal oxide by oxidizing the metal oxide intermediate.
[0009] In yet another embodiment, a system comprising a Fischer-Tropsch reactor is provided. The Fischer-Tropsch reactor is configured to produce hydrocarbon fuel from a feed mixture comprising gaseous fuel. The system also comprises a first reactor comprising a plurality of ceramic composite particles, wherein the ceramic composite particles comprise at least one metal oxide disposed on a support. The first reactor is configured to reduce the metal oxides with a gaseous fuel to a reduced metal or a reduced metal oxide, wherein the gaseous fuel comprises at least partially the hydrocarbon fuel produced by the Fischer-Tropsch reactor. The system also comprises a second reactor configured to oxidize the reduced metal or reduced metal oxide with steam to produce metal oxide intermediates.
[0010] In another embodiment, a method of preparing ceramic composite particles is provided. The method comprises reacting a metal oxide with a support material; heat treating the mixture of metal oxide and support material at temperatures of between about 200 to about 1500 <0>C to produce ceramic composite powders; converting the ceramic composite powders into ceramic composite particles; and reducing and oxidizing the ceramic composite particles prior to use in a reactor...
HIGH PURITY, HIGH PRESSURE HYDROGEN PRODUCTION WITH IN-SITU CO2 AND SULFUR CAPTURE IN A SINGLE STAGE REACTOR
US7837975
[ PDF ]
BACKGROUND AND SUMMARY OF THE INVENTION
[0003] The disclosed embodiments includes a process for producing hydrogen, comprising the steps of: (a) gasifying a fuel into a raw synthesis gas comprising CO, hydrogen, steam and sulfur and halide contaminants in the form of H2S, COS and HX, where X is a halide; (b) passing the raw synthesis gas through a water gas shift reactor (WGSR) into which CaO and steam are injected, the CaO reacting with the shifted gas to remove CO2, sulfur and halides in a solid-phase calcium-containing product comprising CaCO3, CaS and CaX2; (c) separating the solid-phase calcium-containing product from an enriched gaseous hydrogen product; and (d) regenerating the CaO by calcining the solid-phase calcium-containing product at a condition selected from the group consisting of: in the presence of steam, in the presence of CO2, in the presence of synthesis gas, in the presence of H2 and O2, under partial vacuum, and combinations thereof.
[0004] The fuel could be coal, biomass, oil sands, coke, tar, wax oil shales, or combinations of these materials.
[0005] Although the steam may be injected into the WGSR in any functional quantity, it is preferred that the steam injected is in the range of from about the stoichiometric requirement to about 3 times the stoichiometric requirement.
[0006] In one embodiment, the enriched hydrogen product has a purity of at least 60%. In one embodiment, the H2:CO ratio of the enriched hydrogen product is in the range of from about 0.5:1 to about 1000:1. In some embodiments the enriched hydrogen product has a purity in the range of from about 70% to about 99.99%, at temperature in the range of from about 400-1000 C, and a pressure in the range of from about 1 to about 100 atmospheres.
[0007] The WGSR may be of a type selected from the group consisting of: fixed bed reactors, fluidized bed reactors, entrained flow reactors, moving bed reactors rotary kilns, or combinations thereof. Additionally, the calcinations step may be performed in a calcinations reactor of a type selected from the group consisting of: fixed bed reactors, fluidized bed reactors, entrained flow reactors, moving bed reactors rotary kilns, or combinations thereof.
[0008] In some embodiments, the WGSR does not have a catalyst disposed therein. As such the WGSR operates at a temperature in the range of from about 550-750 C, in the pressure range of from about 1 to about 60 atm, it is preferred that the WGSR reactor operate in a temperature range of from about 600-700 C and at a pressure in the range of from about 20 to about 30 atm. In some embodiments, the enriched hydrogen product is 99% pure when 3 times the stoichiometric steam requirement is used. At the stoichiometric steam requirement the process produces an enriched hydrogen product that is 90% pure. In another catalytic embodiment, the enriched hydrogen product has a H2/Co ration of at least 2.5 and a maximum sulfur (H2S/COS) concentration of less than 10 ppm using only the stoichiometric requirement of steam.
[0009] In some embodiments, a catalyst may be used in the WGSR. A suitable high temperature shift catalyst which may include: Fe, Cu, Co, Mo, W, Cs, Pt, Ph, Pd, and other precious metal catalysts or their oxides or sulfides or combinations thereof. Suitable supports for use with the foregoing high temperature shift catalysts include: Cr2O3, ZnO, MgO, ceria, alumina, silica, zirconia and combinations thereof.
[0000] A WGSR reactor with a catalyst operates in the temperature range of from about 550-750 C and at a pressure in the range of from about 1 to about 100 atm. It is preferred that the WGSR reactor operate in the temperature range of from about 600-700 C and at a pressure of from about 20 to about 30 amt. When a catalyst is used the enriched hydrogen product may achieve 99.99% purity when 3* the stoichiometric requirement of steam is used in the WGSR. The enriched hydrogen product may achieve 98% purity when the stoichiometric requirement of steam is used. Some embodiments may attain a purity of at least 80% with a maximum sulfur (H2S/COS) concentration of less than 10 ppm when 3* the stoichiometric requirement of steam is used and at least 70% purity with a maximum sulfur concentration of less than 1 ppm when the stoichiometric requirement of steam is used.
[0010] The process may also comprise the step of (e) recycling at least a portion of a product stream from a Fischer-Tropsch reactor, fed by the WGSR, so as to introduce a chemical species selected from the group consisting of: methane, C1-C4 hydrocarbons, CO, hydrogen and combinations thereof back into the WGSR.
[0011] In all embodiments, the CaO may have a surface area of at least 12.0 m2/g and a pore volume of at least 0.015 cm3/g, said CaO having a sorption capacity of at least about 70 grams of CO2 per kilogram of CaO.
[0012] The CaO may be provided in any usable form including, but not limited to, pellets, granules, fines, monoliths and combinations thereof. The CaO may be obtained by processing chicken eggshells.
[0013] Although the regeneration of CaO step may be performed any functional process, it is preferred that it is conducted by a process selected from the group consisting of: (a) calcining in the presence of steam and/or CO2 and/or H2 with O2, and/or synthesis gas with O2 and/or under partial vacuum or combinations thereof; (b) a process in which the heat is added to the calciner using steam and a combination of calciner fuel and oxidant; (c) a process in which the calciner fuel is H2 or natural gas or synthesis gas or coal or combinations thereof; (d) a process in which the oxidant is air or oxygen or combinations thereof; (e) a process in which heat is provided to the calciner directly or indirectly; (f) calciner reactor temperatures ranging from about 700-1100 C; and (a process for adjusting the calciner temperature by modifying the CaO to CaCO3 ratio in the calciner. The gas phase product from the calciner may comprise pure CO2 and could also contain trace amounts of H2S.
[0014] The disclosed embodiments also includes a process for producing hydrogen, comprising the steps of: (a) reforming a gaseous hydrocarbon fuel in the presence of CaO and steam to remove CO2, sulfur and halide contaminants in the form of H2S, COS and HX, where X is a halide, in a solid-phase calcium-containing product comprising CaCO3, CaS and CaX2, thereby producing a mixture of CO and hydrogen; (b) separating the solid-phase calcium-containing product from an enriched gaseous hydrogen product; and (c) regenerating the CaO by calcining the solid-phase calcium-containing product at a condition selected from the group consisting of: in the presence of steam, in the presence of CO2, in the presence of synthesis gas, in the presence of H2 and O2, under partial vacuum, and combinations thereof.
[0015] The gaseous fuel may be natural gas, C1-C4 hydrocarbons, or mixtures thereof. The reforming step may involve the introduction of CO2, so called dry reforming.
[0016] The reforming step may involve a reforming catalyst. Suitable reforming catalysts include those comprising: Ni, Pt, Rh, Pd, Ru, W, Mo, their oxide or carbides or sulfides. The reforming catalyst may use a support. Suitable supports for use with the foregoing reforming or pre-reforming catalysts include: alumina, silica, titania, zirconia, and combinations thereof. It is preferred that the reforming catalyst is sulfur intolerant.
[0017] The reforming operation may occur in a temperature range of from about 550 to about 750 C. and at a pressure in the range of from about 1 to about 60 atm. Preferably, it operates in the temperature range of from about 600 to about 70[deg.] C. and at a pressure in the range of from about 20 to about 30 atm.
[0018] The enriched hydrogen product produced may be as pure as 99.9% when 3* the stoichiometric requirement of steam is used and 95% pure when the stoichiometric requirement of steam is used.
[0019] This process may additionally comprise the step of: (d) recycling at least a portion of a product stream from a Fischer-Tropsch reactor, fed by the reformer, so as to introduce a chemical species selected from the group consisting of: methane, C1-C4 hydrocarbons, CO, hydrogen and combinations thereof back into the reformer.
[0020] In all embodiments, the CaO may have a surface area of at least 12.0 m2/g and a pore volume of at least 0.015 cm3/g, said CaO having a sorption capacity of at least about 70 grams of CO2 per kilogram of CaO.
[0021] The CaO may be provided in any usable form including, but not limited to, pellets, granules, fines, monoliths and combinations thereof. The CaO may be obtained by processing chicken eggshells.
[0022] When a catalyst is used the enriched hydrogen product may achieve 99.99% purity when 3* the stoichiometric requirement of steam is used. The enriched hydrogen product may achieve 98% purity when the stoichiometric requirement of steam is used. Some embodiments may attain a purity of at least 80% with a maximum sulfur (H2S/COS) concentration of less than 10 ppm when 3* the stoichiometric requirement of steam is used and at least 70% purity with a maximum sulfur concentration of less than 1 ppm when the stoichiometric requirement of steam is used. The process allows for a hydrogen purity of at least 80% with a maximum sulfur (H2S/COS) concentration of less than 10 ppm when 3* the stoichiometric requirement of steam is used and at least 70% purity with a maximum sulfur concentration of less than 1 ppm when the stoichiometric requirement of steam is used.
[0023] Another process of the disclosed embodiments for producing hydrogen, comprising the steps of: (a) at least partially oxidizing a fuel into a raw gas comprising CO, hydrogen, steam and sulfur and halide contaminants in the form of H2S, COS and HX, where X is a halide; (b) passing the raw gas through a water gas shift reactor (WGSR) into which CaO and steam are injected, the CaO reacting with the shifted gas to remove CO2, sulfur and halides in a solid-phase calcium-containing product comprising CaCO3, CaS and CaX2; (c) separating the solid-phase calcium-containing product from an enriched gaseous hydrogen product; and (d) regenerating the CaO by calcining the solid-phase calcium-containing product at a condition selected from the group consisting of: in the presence of steam, in the presence of CO2, in the presence of synthesis gas, in the presence of H2 and O2, under partial vacuum, and combinations thereof.
[0024] In all embodiments, the CaO may have a surface area of at least 12.0 m2/g and a pore volume of at least 0.015 cm3/g, said CaO having a sorption capacity of at least about 70 grams of CO2 per kilogram of CaO.
[0025] The CaO may be provided in any usable form including, but not limited to, pellets, granules, fine, monoliths and combinations thereof. The CaO may be obtained by processing chicken eggshells.
[0026] Although the steam may be injected into the WGSR in any functional quantity, it is preferred that the steam injected is in the range of from about the stoichiometric requirement to about 3 times the stoichiometric requirement.
[0027] The WGSR may be of a type selected from the group consisting of: fixed bed reactors, fluidized bed reactors, entrained flow reactors, moving bed reactors rotary kilns, or combinations thereof. Additionally, the calcinations step may be performed in a calcinations reactor of a type selected from the group consisting of: fixed bed reactors, fluidized bed reactors, entrained flow reactors, moving bed reactors rotary kilns, or combinations thereof.
[0028] In some embodiments, the WGSR does not have a catalyst disposed therein. As such the WGSR operates at a temperature in the range of from about 550-750 C, in the pressure range of from about 1 to about 60 atm, it is preferred that the WGSR reactor operate in a temperature range of from about 600-700 C and at a pressure in the range of from about 20 to about 30 atm. In some embodiments, the enriched hydrogen product is 99% pure when 3 times the stoichiometric steam requirement is used. At the stoichiometric steam requirement the process produces an enriched hydrogen product that is 90% pure. In another catalytic embodiment, the enriched hydrogen product has a H2/Co ratio of at least 2.5 and a maximum sulfur (H2S/COS) concentration of less than 10 ppm using only the stoichiometric requirement of steam.
[0029] In some embodiments, a catalyst may be used in the WGSR. A suitable high temperature shift catalyst which may include: Fe, Cu, Co, Mo, W, Cs, Pt, Ph, Pd, and other precious metal catalysts or their oxides or sulfides or combinations thereof. Suitable supports for use with the foregoing high temperature shift catalysts include: Cr2O3, ZnO, MgO, ceria, alumina, silica, zirconia and combinations thereof.
[0030] A WGSR reactor with a catalyst operates in the temperature range of from about 550-750 C and at a pressure in the range of from about 1 to about 100 atm. It is preferred that the WGSR reactor operate in the temperature range of from about 600-700 C and at a pressure of from about 20 to about 30 atm. When a catalyst is used the enriched hydrogen product may achieve 99.99% purity when 3* the stoichiometric requirement of steam is used in the WGSR. The enriched hydrogen product may achieve 98% purity when the stoichiometric requirement of steam is used. Some embodiments may attain a purity of at least 80% with a maximum sulfur (H2S/COS) concentration of less than 10 ppm when 3* the stoichiometric requirement of steam is used and at least 70% purity with a maximum sulfur concentration of less than 1 ppm when the stoichiometric requirement of steam is used...
REFERENCES AND PRIOR ART
[0000] Adanez, J.; Garcia-Labiano, F.; Abad, A.; de Diego L. F.; Gayan, P. "Regeneration of Sulfided Dolomite with Steam and Carbon Dioxide". Energy and Fuels. 2001, 15, 85-94.
Agnihotri, R.; Mahuli, S. K.; Chauk, S. S.; Fan, L-S. "Influence of Surface Modifiers on the Structure of Precipitated Calcium Carbonate". Ind. Eng. Chem. Res. 1999, 38, 2283-2291.
Balasubramanian, B.; Lopez-Ortiz, A.; Kaytakoglu, S.; Harrison D. P. "Hydrogen from Methane in a Single-Step Process". Chem. Engng. Sci., 1999, 54, 3543-3552.
Barker, R. "The Reversibility of the Reaction CaCO3=CaO+CO2". J. Appl. Chem. Biotechnol. 1973, 23, 733-742.
Beruto, D.; Searcy, A. W. "Calcium Oxides of High Reactivity." Nature. 1976, 263, 221-222.
Beruto, D.; Barco, L.; Searcy, A. W.; and Spinolo, G. "Characterization of the Porous CaO Particles Formed by Decomposition of CaCO3 and Ca(OH)2 in Vacuum". J. Am. Cer. Soc. 1980, 63, 439-443.
Bohlbro H., "An Investigation on the Kinetics of Conversion of Carbon Monoxide with Water Vapour over Iron Oxide Based Catalysts". Second Edition, Haldor Topsoe, Denmark (1969).
Chauk, S. S.; Agnihotri, R.; Jadhav R. A.; Misro S. K.; Fan, L-S. "Kinetics of High-Pressure Removal of Hydrogen Sulfide Using Calcium Oxide Powder". AlChE J. 2000, 46, 1157-1167.
David N. S. "The Water-Gas Shift Reaction". Catal. Rev. Sci. Eng. 1980, 21, 275-318.
Dash, S.; Kamruddin, M.; Ajikumar, P. K.; Tyagi, A. K.; Raj, B. "Nanocrystalline and Metastable Phase Formation in Vacuum Thermal Decomposition of Calcium Carbonate". Thermochimica acta. 2000, 363, 129-135.
Doong, Shain; Ong, Estela; Atroshenko, Mike; Lau, Francis; Roberts, Mike. "A Novel Membrane Reactor for Direct Hydrogen Production from Coal". DOE Final Technical Report. January 2006. http://www.osti.gov/bridge/servlets/purl/876470-v2h bxY/876470. PD F.
Fan, L-S.; Ghosh-Dastidar, A.; Mahuli, S. "Calcium Carbonate Sorbent and Methods of Making and Using Same". U.S. Pat. No. 5,779,464, Jul. 14 (1998).
Fan, L-S.; Jadhav R. A. "Clean Coal Technologies: OSCAR and CARBONOX Commercial Demonstrations". AlChE J. 2002, 48, 2115-2123.
Gerhartz W., "Ullmann's Encyclopedia of Industrial Chemistry", A12, 5thedn., VCH, New York pp. 179-242 (1993).
Ghosh-Dastidar, A.; Mahuli, S. K.; Agnihotri, R.; Fan, L-S. "Investigation of High-Reactivity Calcium Carbonate Sorbent for Enhanced SO2 Capture". Ind. Eng. Chem. Res. 1996, 35, 598-606.
Gupta, H.; Fan, L-S. "Carbonation-Calcination Cycle Using High Reactivity Calcium Oxide for Carbon Dioxide Separation from Flue Gas", Ind. Eng. Chem. Res. 2002, 41, 4035-4042.
Gupta, H; Iyer, M. V.; Sakadjian, B. B.; and Fan, L.-S., Proceedings from Fuel Cell Seminar, San Antonio, Tex., 2004.
Hufton, J. R.; Mayorga, S.; Sircar, S. "Sorption-Enhanced Reaction Process for Hydrogen Production." AlChE J. 1999, 45, 248-256.
Iyer, M. V.; Gupta, H.; Sakadjian, B. B.; Fan, L.-S. "Multicyclic Study on the Simultaneous Carbonation and Sulfation of High-Reactivity CaO". Ind. Eng. Chem. Res. 2004, 43, 3939-3947.
Kato, M.; Yoshikawa, S.; Nakagawa, K. "Carbon Dioxide Absorption by Lithium Orthosilicate in a Wide Range of Temperature and Carbon Dioxide Concentrations". J. Mat. Sci. Lett. 2002, 21, 485-487.
Lin, Shi-Ying; Suzuki, Yoshizo; Hatano, Hiroyuki; Harada, Michiaki. "Developing an Innovative Method, HyPr-RING, to Produce Hydrogen from Hydrocarbons." Energy Conversion and Management. 2002, 43, 1283-1290.
Lin S.; Harada M. Suzuki Y.; Hatano H. "Process Analysis for Hydrogen Production by Reaction
Integrated Novel Gasification (HyPr-RING)". Energy Conv. Mgmt. 2005, 46, 869-880.
Lopez-Ortiz, A.; Harrison D. P. "Hydrogen Production Using Sorption Enhanced Reaction". Ind. Eng. Chem. Res. 2001, 40, 5102-5109.
Nakagawa, K. "Lithium Silicates for the Separation of CO2 from Flue Gas". Carbon Dioxide Capture Workshop at NETL, Pittsburgh, February (2003).
Ortiz, A. L.; Harrison D. P. "Hydrogen Production Using Sorption Enhanced Reaction". Ind. Eng. Chem. Res., 2001, 40, 5102-5109.
Rosen, M. A. "Thermodynamic Comparison of Hydrogen Production Processes", Int. J. Hydrogen Energy. 1996, 21, 349-365.
Rosen, M. A.; Scott, D. S. "Comparative Efficiency Assessments for a Range of Hydrogen Production Processes". Int. J. Hydrogen Energy. 1998, 23, 653-659.
Roark, S. E.; Mackay, R.; Sammells, A. F. "Hydrogen Separation Membranes for Vision 21 Energy Plants". Proceedings of the International Technical Conference on Coal Utilization & Fuel Systems. 2002, 27 (Vol. 1), 101-112.
Ruth, L. A.; Varga, G. M. Jr. "Developing Regenerable Sulfur Dioxide Sorbents for Fluidized Bed Coal Combustion Using Thermogravimetric Analysis". Thermochimica Acta. 1978, 26, 241-255.
Stiegel, Gary J.; Ramezan, Massood. "Hydrogen from Coal Gasification: An Economical Pathway to a Sustainable Energy Future". International Journal of Coal Geology. 2006, 65, 173-190.
Turkdogan, E. T.; Rice, B. B. "Desulfurization of Limestone and Burnt Lime" Trans. Society of Min. Eng of AIME. 1973, 254, 28-33.
Wei, S-H.; Mahuli, S. K.; Agnihotri, R.; Fan, L-S. "High Surface Area Calcium Carbonate: Pore Structural Properties and Sulfation Characteristics". Ind. Eng. Chem. Res. 1997, 36, 2141-2148.
White, C. M.; Strazisar, B. R.; Granite, E. J.; Hoffman, J. S.; Pennline, H. W. "Separation and Capture of CO2 from Large Stationary Sources and Sequestration in Geological Formations-Coalbeds and Deep Saline Aquifers". J. Air & Waste Manage. Assoc. 2003, 53, 645-715.
Wu, S.; Uddin, M. A.; Su, C.; Nagamine, S.; Sasaoka, E. "Effect of Pore-Size Distribution of Lime on the Reactivity for the Removal of SO2 in the Presence of High-Concentration CO2 at High Temperature". Ind. Eng. Chem. Res. 2002, 41, 5455-5458.
Ziock, H.-J; Lackner, K. S.; Harrison, D. P. "Zero Emission Coal Power, a New Concept." http://www.netl.doe.gov/publications/proceedings/01/carbon_seq/2b2.pdf.
A system for converting fuel is provided and includes a first reactor comprising a plurality of ceramic composite particles, the ceramic composite particles comprising at least one metal oxide disposed on a support, wherein the first reactor is configured to reduce the at least one metal oxide with a fuel to produce a reduced metal or a reduced metal oxide; a second reactor configured to oxidize at least a portion of the reduced metal or reduced metal oxide from the said first reactor to produce a metal oxide intermediate; a source of air; and a third reactor communicating with said source of air and configured to regenerate the at least one metal oxide from the remaining portion of the solids discharged from the said first reactor and the solids discharged from the said second reactor by oxidizing the metal oxide intermediate.
The present invention is generally directed to systems and methods of converting carbonaceous fuels. Reduction- Oxidation (redox) reactions, with the presence of one or more chemical intermediates, are generally utilized to convert the carbonaceous fuels.
In order to meet the ever increasing demand for clean and affordable energy carriers and to ensure the sustainable growth of modern economy, efficient and environmentally friendly technologies that convert carbonaceous fuels such as coal, crude oil, natural gas, biomass, tar sands, and oil shale into carbon free energy carriers are highly desirable. An energy carrier is a substance or phenomenon that can be used to produce mechanical work or heat or to operate chemical or physical processes.
Existing carbonaceous fuel conversion technologies are either capital intensive (gasification or ultra-supercritical pulverized coal combustion), have low efficiencies (sub- critical pulverized coal combustion), or both, especially when CO2 regulation is mandatory.
Chemical reactions between carbonaceous fuels and air/steam/CO2 through the assistance of a metal oxide medium may represent an effective way to convert the fuels. A number of techniques have been proposed to convert carbonaceous fuels using metal oxide. For example, Watkins, U.S. Patent No. 3,027,238, describes a method for producing hydrogen gas including reducing a metal oxide in a reducing zone, and oxidizing the reduced metal with steam to produce hydrogen in an oxidizing zone. Thomas et al., U.S. Published Application No. 2005/0175533, and Fan et al., PCT Application No. WO 2007/082089, both describe methods for producing hydrogen gas by reducing a metal oxide in a reduction reaction between a carbon-based fuel and a metal oxide to provide a reduced metal or metal oxide having a lower oxidation state, and oxidizing the reduced metal or metal oxide to produce hydrogen and a metal oxide having a higher oxidation state. The metal or metal oxide is provided in the form of a porous composite of a ceramic material containing the metal or metal oxide. A well known process is a steam-iron process wherein coal-derived producer gas is reacted with iron oxide particles to be later regenerated with steam to produce hydrogen gas. However, a fluidized bed is used in this system which causes iron (Fe) to loop between FeO and Fe3U4, the gas is not fully converted, and no pure gas stream can be produced. Ishida et al., U.S. Patent No. 5,447,024, describes processes that make use of nickel oxide particles to convert natural gas through a chemical looping process into heat to be used in a turbine. However, this technology has limited applicability because it can only convert costly natural gas into heat/electricity. Therefore, both the feedstock and the product of the process are restricted.
With increasing demand for cleaner and more efficient energy carriers such as electricity, hydrogen, and fuels, the need arises for improved systems, and system components therein, which produce the aforementioned energy carriers with higher efficiency and lower emissions.
Embodiments of the present invention provide novel systems and processes for converting solid, liquid, and gaseous fuels into efficient energy carriers. In one embodiment, a system for converting solid, liquid, or gaseous fuel is provided and comprises a first reactor comprising a plurality of ceramic composite particles. The ceramic composite particles comprise at least one metal oxide disposed on a support, and the first reactor is configured to reduce the at least one metal oxide with a fuel to produce a reduced metal or a reduced metal oxide. The system includes a second reactor configured to at least partially re-oxidize the reduced metal or reduced metal oxide to produce a metal oxide intermediate. The system also includes a source of air and a third reactor communicating with the source of air and configured to regenerate the at least one metal oxide by oxidizing the metal oxide intermediate. In a preferred form, the fuel is a solid fuel or a gaseous fuel. Optionally, a fuel conversion enhancement gas, preferably including CO2, steam, and/or H2, is sent to the first reactor in which the gas flows countercurrently to the flow of solids. Also provided is a method of preparing ceramic composite particles, for example in the form of pellets, comprising the steps of, mixing a metal oxide with at least one ceramic material to form a mixture, granulating the mixture, and drying the granulated mixture. The dried, granulated mixture is processed into particle form such that the characteristic length of the particles is greater than about 200 [mu]m. The particles are heat treated at a temperature of from about 500 to about 1500<0>C and optionally may be reduced and oxidized prior to use in the reactor system.
METHODS AND SYSTEMS FOR SYNTHESIZING IRON-BASED MATERIALS AND SEQUESTERING CARBON DIOXIDE
WO2010132784
[ PDF ]
Methods and systems for sequestering carbon dioxide and generating hydrogen are disclosed. In some embodiments, the methods include the following: dissolving an iron based material that includes a carbonate-forming element into a solution including the carbonate-forming element and iron; increasing a pH of the solution to cause precipitation of iron oxide from the solution thereby generating a first source of Fe2O3; reacting the carbonate-forming element in the solution with a first source of carbon dioxide to produce a carbonate thereby sequestering the carbon dioxide; oxidizing the first source of Fe2O3 with a carbonaceous fuel thereby generating a second source of carbon dioxide and iron; and oxidizing the iron with steam thereby generating hydrogen and an iron oxide. Some embodiments include producing iron-based catalysts.
BACKGROUND
[0002] Since the industrial revolution, the amount of CO2 in the atmosphere has risen from 280 ppm in 1800 to 370 ppm in 2000, mainly due to the consumption of fossil fuels. More than half of the energy used in the United States comes from the use of coal, and it is mostly used to generate electricity. Unfortunately, CO2 is one of the greenhouse gases considered to be responsible for global warming. Moreover, the increased atmospheric CO2 concentration will acidify the ocean and will change the chemistry of the surface ocean, leading to a potentially detrimental impact on the ecosystem. In order to meet the ever- increasing global energy demands, while stabilizing the atmospheric CO2 level, current carbon emissions should be significantly reduced.
[0003] There have been significant research and development activities in the area of carbon capture and storage (CCS), including a number of integrated technologies (e.g., chemical looping processes) to combine CO2 capture with electricity/chemical/fuel production. Chemical looping processes involve a sorbent, typically a metal, or more likely a low oxidation state metal oxide that can be oxidized in air. The oxide is reduced by carbonaceous fuels in a subsequent step. A variation of this approach oxidizes the metal not in air but in a chemical reaction with steam to produce a pure stream of H2. The chemical looping processes also allow the inherent generation of the sequestration-ready CO2 stream at higher pressures.
[0004] Once captured, CO2 can be stored via geological sequestration, ocean disposal, mineral carbonation, and biological fixation. The mineral sequestration scheme is particularly attractive, since this process converts CO2 into thermodynamically stable carbonates via the reaction of CO2 with widely available non-carbonate minerals, such as serpentine and olivine. Therefore, the mineral sequestration process eliminates the risk of accidental CO2 releases. The reaction underlining mineral carbonation mimics natural chemical transformations of CO2, such as the weathering of rocks. The main challenges of this storage method have been the slow dissolution kinetics and large energy requirement associated with the mineral processing.
SUMMARY
[0005] The previously developed pH swing carbon mineral sequestration immobilizes the gaseous CO2 into a thermodynamically stable solid, MgCO3, using Mg-bearing minerals such as serpentine. This mineral carbonation technology is particularly promising since it generates value-added solid products: high surface area silica, iron oxide, and magnesium carbonate, while providing a safe and permanent storage option for CO2. By carefully controlling the pH of the system, these solids products can be produced with high purity. The disclosed subject matter focuses on the synthesis of iron oxide particles as a chemical looping sorbent in order to achieve the integration between carbon capture and storage technologies. The synthesized iron-based chemical looping sorbent has been found to be as effective as commercially available iron oxide nanoparticles at converting syngas/carbonaceous fuel into high purity H2, while producing a sequestration-ready CO2 stream.
[0006] The disclosed subject matter utilizes the iron component of magnesium-bearing minerals, e.g., olivine and serpentine, during carbon mineral sequestration. These minerals often contain 5-10 percent by weight of iron, and the recovery and utilization of iron during the mineral processing increases the economic feasibility of carbon mineral sequestration technology. Among many applications of iron-based materials, the disclosed subject matter focuses on the synthesis of iron-based chemical looping sorbents, which can be used for carbon dioxide capture and hydrogen production, as well as the syntheses of iron-based catalysts to be used in the production of synthetic liquid fuels and hydrogen from carbonaceous materials including coal, biomass, and municipal solid wastes...
CALCIUM LOOPING PROCESS FOR HIGH PURITY HYDROGEN PRODUCTION INTERGRATED WITH CAPTURE OF CARBON DIOXIDE, SULFUR AND HALIDE
WO2010045232
[ PDF ]
A process for producing hydrogen comprising the steps of: (i) gasifying a fuel into a raw synthesis gas comprising CO, hydrogen, steam, sulfur and halide contaminants in the form of H2S, COS, and HX, wherein X is a halide; (ii) passing the raw synthesis gas through a water gas shift reactor (WGSR) into which CaO and steam are injected, the CaO reacting with the shifted gas to remove CO2, sulfur and halides in a solid-phase calcium-containing product comprising CaCO3, CaS and CaX2; (iii) separating the solid-phase calcium-containing product from an enriched gaseous hydrogen product; and (iv) regenerating the CaO by calcining the solid-phase calcium-containing product at a condition selected from the group consisting of: in the presence of steam, in the presence of CO2, in the presence of synthesis gas, in the presence of H2 and O2, under partial vacuum, and combinations thereof.
BACKGROUND OF THE ART AND SUMMARY OF EXEMPLARY EMBODIMENTS OF THE INVENTION
[0004] The production of gaseous hydrogen, and particularly, gaseous hydrogen of high purity, is known in the prior art. A variety of feedstocks are known to be useful for these processes, including petroleum, coal, biomass, oil sands, coke, tar, wax oil shales, or combinations of these materials. Depending upon the feedstock selected, the amount of sulfur and halogens present in the feedstock can vary extensively, and many considerations, including catalyst poisoning and the cost of environmental control equipment can be effected by these specific contaminants. [0005] Also, the process used will affect the amount of carbon dioxide produced. As carbon dioxide is associated with global warming, emissions of carbon dioxide must be controlled. [0006] It is therefore an unmet advantage of the prior art to provide a process of this type wherein the carbon dioxide, sulfur and halides are captured as a part of the hydrogen production process.
[0007] The rising energy demand coupled with the depleting global oil reserves and the environmental degradation due to emissions has led to extensive research in the field of clean energy production. The total energy use, globally, has been predicted to increase from 421 quadrillion BTU in 2003 to 722 quadrillion BTU in 2030. <l> In the United States, the annual energy consumption is projected to increase by 71% from 2003 to 2030, which is much higher than the predicted increase in the domestic energy production. Currently, the United States is dependent on foreign oil for 56% of its energy needs. This translates to the fact that although the production capacity of petroleum products and natural gas will increase, the US will be dependent on foreign oil for 70% of its energy needs by 2025. <l> On the other front, the energy related CO2 emission has increased at an annual average percentage of 1.3 % in the past decade and is projected by the EIA to increase at the same rate till 2030. To add to this, oil prices are expected to soar up by 50 % at the end of 2030. <l> Hence, the implementation of energy generation technologies as well as production of "Green" fuels which will reduce the dependence on oil, limit the emissions of CO2, sulfur and other pollutants and be economically feasible are the need of the hour.
[0008] This need has led to a global push towards the development of efficient, economical, and reliable carbon capture and sequestration technologies (CCS) for application to fossil fuel based power plants. Coal is present in abundance, about 494 billion tons of reserves in the United States, within which the state of Ohio has 5% or 24 billion tons of reserves. While it gives rise to harmful emissions it can be used to provide a major portion of our energy needs if CCS is implemented in a carbon constrained scenario. The implementation of CO2 capture could be through post combustion capture, oxy-combustion and pre-combustion. These technologies could be applied to either coal, natural gas or biomass based systems. Figure 1 illustrates these concepts through simplified flow diagrams.
[0009] Post combustion capture technology involves the combustion of coal or natural gas to produce hot flue gas which is used to generate steam. The CO2 from the flue gas is then captured using solvents or sorbents. Although coal combustion for power generation is economically viable in a non - carbon constrained scenario, this will not be true when a CO2 regulation is applied. This is because the combustion of coal or natural gas results in the production of large volumes of flue gas in which the CO2 concentration is very low (13-14% for coal combustion and 3-4% for natural gas combustion) and hence the capture of CO2 becomes inefficient and expensive. Addition of CO2 capture results in plant efficiency losses of 8-12 % resulting in a net efficiency of 35% for a Super Critical Pulverized Coal Combustion (SC-PCC) plant on an LHV basis.<2> In oxy-combustion, the fuel is burnt in oxygen and recycled flue gas, to produce a concentrated stream containing CO2 and H2O which is then dried, compressed and transported for sequestration. Although oxy-combustion obviates the need for a separate CO2 capture stage, it requires an Air Separation Unit (ASU) which is energy intensive and expensive. Oxy-combustion also yields in an overall LHV efficiency of 35 % for an SC-PCC plant similar to the post combustion capture case.<2> Pre combustion capture involves the gasification of coal or the reforming of natural gas to produce syngas. The syngas is then cleaned and sent to shift reactors (WGSR) to convert the carbon monoxide to H2 and CO2 in the presence of steam. The CO2 is then captured from the shifted syngas and the H2 is either combusted to produce electricity or purified in a Pressure Swing Absorber (PSA) and used for the production of chemicals and liquid fuels. The overall efficiency of an IGCC plant with CO2 capture is 38-40% which is higher than that for post combustion and oxy-combustion systems.<2>
[0010] Pre-combustion capture technologies are a potential solution to efficient and economical CCS implementation as gasification results in the production of a lower level of criteria pollutants when compared to combustion and the application of CCS to gasification systems is more efficient and economical when compared to CCS for post combustion systems. It has been estimated that with the implementation of CCS using solvent based systems, the increase in the COE for an IGCC is 25 to 40 % while that for PC boilers is 60 to 85%. In a carbon constrained scenario, it has been estimated that the cost of a super critical PC boiler will be $2140/KWe while that of an IGCC will be $1890/KWe. In addition to being more economical and efficient, gasification is also very versatile and capable of producing H2 and liquid fuels in addition to electricity.<3>
[0011] Applying CO2 capture to coal gasification requires the addition of shift reactors, a CO2 separation process and CO2 compression and drying. In a typical gasification system, coal is partially oxidized in the presence of steam and oxygen to produce syngas which is then converted to H2, electricity or liquid fuels.
[0012] Coal Gasification: CxHy + H2O = xCO + (Vi + I) H2 (1)
[0013] For the implementation of CCS, the CO in syngas needs to be converted to H2 and CO2 via the WGS reaction so that a large fraction of the carbon content can be captured.
[0014] WGS reaction: CO + H2O = CO2 + H2 (2)
[0015] In the conventional scenario, a series of shift reactors with catalysts and excess steam addition is used due to the thermodynamic limitation of the WGS reaction. Depending on the sulfur tolerance of the catalyst, the WGSR can be conducted as a raw syngas (sour) shift or the clean syngas (sweet) shift. Commercially the clean WGSR is carried out in two stages: the high and low temperature shift reactors using iron oxide and copper catalysts respectively. The high temperature shift is conducted to convert the bulk of the carbon monoxide to H2 due to the fast kinetics. The lower temperature shift reaction is carried out as the equilibrium conversion is higher at lower temperatures but the gas stream has to be cooled down to 210 C-240 C which makes the process, energy inefficient.<4> Further, the commercial iron oxide catalyst has a sulfur tolerance of only about 100 ppm and the copper catalyst has a lower tolerance to sulfur (<0.1 ppm) and chloride impurities. Hence syngas clean up is required upstream of the shift reactors to remove sulfur, chloride and other impurities and downstream of the shift operation to remove CO2. Cleanup is achieved using conventional scrubbing technology which is energy intensive due to the cooling and heating requirements. The sour gas shift uses a sulfided catalyst which is resistant to high sulfur concentrations in the syngas stream and operates at a temperature of 250- 500C. By using the raw gas shift, sulfur removal and CO2 removal can be conducted down stream of the shift reactor in an integrated mode. However the extent of CO conversion is lower in the raw gas shift than in the clean gas shift. Addition of the CO2 capture step results in a 25 - 40% increase in the cost of electricity (COE), 7.2% decrease in the efficiency, 2.1% due to CO2 compression and 0.9% due to CO2 capture.<3>
[0016] Conventional pre-combustion capture in a natural gas based plant involves methane reforming which is conducted at temperatures greater than 900C and is highly energy intensive.<5>
[0017] Steam Methane Reforming (SMR): CH4 + H2O = CO + 3H2 (3)
[0018] The syngas obtained is then shifted similar to the operation in the IGCC system and CO2 capture is achieved at low temperatures using physical (eg. selexol, rectisol, chilled ammonia) or chemical (eg. amine solutions) solvents resulting in a large increase in the parasitic energy requirement and related cost of energy. Hence there is a need to improve the energy efficiency and economics by implementing process intensification to reduce the foot print and improve the heat integration within the system. [0019] The Calcium Looping Process (CLP) developed at the Ohio State University<6>, as illustrated in Figures 2 and 3, improves the efficiency of the coal/natural gas to H2 process by integrating various unit operations into a single stage. The CLP not only aids in curbing CO2 emissions but also improves the efficiency and reduces the CO2 foot print. It utilizes a high temperature regenerable CaO sorbent which in addition to capturing the CO2, enhances the yield of H2 and simultaneously captures sulfur and halide impurities. It also enhances the yield of liquid fuels by reforming the lighter hydrocarbons and unconverted syngas into hydrogen which is used to increase the H2:C0 ratio in the syngas to 2 and for hydrotreating the liquid fuel. [0020] Figure 2 depicts the integration of the CLP in a coal gasification system. Syngas obtained from coal gasification is sent through a particulate capture device to the integrated H2 production reactor. When CaO is injected into the syngas it reacts with the CO2, H2S, COS and HCl to form a mixture containing predominantly CaCO3 and small amounts of calcium sulfide and calcium chloride. The insitu removal of CO2 removes the equilibrium limitation of the WGS reaction thereby obviating the need for a catalyst and excess steam addition. The CaCO3 is subsequently calcined to yield a pure CO2 stream for sequestration and the CaO is recycled back. In this process, naturally occurring limestone which is cheap and abundantly available is used and its capture capacity is maintained at 12.5 moles CO2/Kg of CaO over multiple cycles which is significantly larger than other solvents and sorbents. Thus the CLP integrates several unit operations, such as the WGSR, CO2 capture system, sulfur removal and halide removal in one process module. Figure 3 shows the integration of the CLP in a natural gas reforming process in which the unit operations namely, reforming, WGS, CO2 capture and sulfur removal are integrated in a single reactor system. Within the H2 production reactor, the natural gas is reformed with steam in the presence of the reforming catalyst and CaO sorbent. The removal of CO2 removes the thermodynamic limitation of the WGSR and the reforming reaction and results in a high conversion of the methane to H2. The H2 production reactor is heat neutral due to the exothermic energy from the WGS and carbonation reactions being equal to the endothermic reforming reaction heat duty. Hence the temperature of operation for the reforming reaction can be reduced from over 900C to 650C. The spent sorbent containing CaCO3, CaO and CaS is separated from the H2 and regenerated in a calciner to produce a sequestration ready CO2 stream. The CaO sorbent is then recycled back to the integrated H2 production reactor. [0021] The overall objectives of the CLP are to improve process efficiency and economics by process intensification, produce H2 for electricity generation, chemicals and liquid fuels synthesis with integrated carbon and contaminants capture at high temperatures, produce a sequestration ready CO2 stream, reduce excess steam requirement and obviate the need for a WGS catalyst. Experimental investigation in a bench scale facility reveals that high purity H2 of 99.7% purity with less that 1 ppm sulfur impurity can be produced. Process evaluation using ASPEN Plus(R) software suggests that the overall efficiency of the coal to H2 process integrated with the CLP is 64% (HHV) which is significantly higher than 57% (HHV) achieved by the state-of-the-art H2 from coal process...
METHODS AND SYSTEMS FOR SYNTHESIZING IRON-BASED MATERIALS AND SEQUESTERING CARBON DIOXIDE
WO2010132784
[ PDF ]
Methods and systems for sequestering carbon dioxide and generating hydrogen are disclosed. In some embodiments, the methods include the following: dissolving an iron based material that includes a carbonate-forming element into a solution including the carbonate-forming element and iron; increasing a pH of the solution to cause precipitation of iron oxide from the solution thereby generating a first source of Fe2O3; reacting the carbonate-forming element in the solution with a first source of carbon dioxide to produce a carbonate thereby sequestering the carbon dioxide; oxidizing the first source of Fe2O3 with a carbonaceous fuel thereby generating a second source of carbon dioxide and iron; and oxidizing the iron with steam thereby generating hydrogen and an iron oxide. Some embodiments include producing iron-based catalysts.
BACKGROUND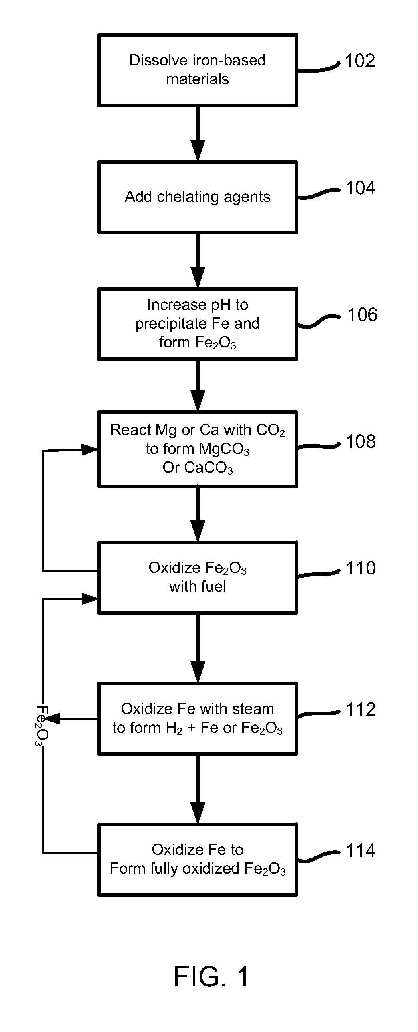
[0002] Since the industrial revolution, the amount of CO2 in the atmosphere has risen from 280 ppm in 1800 to 370 ppm in 2000, mainly due to the consumption of fossil fuels. More than half of the energy used in the United States comes from the use of coal, and it is mostly used to generate electricity. Unfortunately, CO2 is one of the greenhouse gases considered to be responsible for global warming. Moreover, the increased atmospheric CO2 concentration will acidify the ocean and will change the chemistry of the surface ocean, leading to a potentially detrimental impact on the ecosystem. In order to meet the ever- increasing global energy demands, while stabilizing the atmospheric CO2 level, current carbon emissions should be significantly reduced.
[0003] There have been significant research and development activities in the area of carbon capture and storage (CCS), including a number of integrated technologies (e.g., chemical looping processes) to combine CO2 capture with electricity/chemical/fuel production. Chemical looping processes involve a sorbent, typically a metal, or more likely a low oxidation state metal oxide that can be oxidized in air. The oxide is reduced by carbonaceous fuels in a subsequent step. A variation of this approach oxidizes the metal not in air but in a chemical reaction with steam to produce a pure stream of H2. The chemical looping processes also allow the inherent generation of the sequestration-ready CO2 stream at higher pressures.
[0004] Once captured, CO2 can be stored via geological sequestration, ocean disposal, mineral carbonation, and biological fixation. The mineral sequestration scheme is particularly attractive, since this process converts CO2 into thermodynamically stable carbonates via the reaction of CO2 with widely available non-carbonate minerals, such as serpentine and olivine. Therefore, the mineral sequestration process eliminates the risk of accidental CO2 releases. The reaction underlining mineral carbonation mimics natural chemical transformations of CO2, such as the weathering of rocks. The main challenges of this storage method have been the slow dissolution kinetics and large energy requirement associated with the mineral processing.
SUMMARY
[0005] The previously developed pH swing carbon mineral sequestration immobilizes the gaseous CO2 into a thermodynamically stable solid, MgCO3, using Mg-bearing minerals such as serpentine. This mineral carbonation technology is particularly promising since it generates value-added solid products: high surface area silica, iron oxide, and magnesium carbonate, while providing a safe and permanent storage option for CO2. By carefully controlling the pH of the system, these solids products can be produced with high purity. The disclosed subject matter focuses on the synthesis of iron oxide particles as a chemical looping sorbent in order to achieve the integration between carbon capture and storage technologies. The synthesized iron-based chemical looping sorbent has been found to be as effective as commercially available iron oxide nanoparticles at converting syngas/carbonaceous fuel into high purity H2, while producing a sequestration-ready CO2 stream.
[0006] The disclosed subject matter utilizes the iron component of magnesium-bearing minerals, e.g., olivine and serpentine, during carbon mineral sequestration. These minerals often contain 5-10 percent by weight of iron, and the recovery and utilization of iron during the mineral processing increases the economic feasibility of carbon mineral sequestration technology. Among many applications of iron-based materials, the disclosed subject matter focuses on the synthesis of iron-based chemical looping sorbents, which can be used for carbon dioxide capture and hydrogen production, as well as the syntheses of iron-based catalysts to be used in the production of synthetic liquid fuels and hydrogen from carbonaceous materials including coal, biomass, and municipal solid wastes...
CARBONATION CALCINATION REACTION PROCESS FOR CO2 CAPTURE USING A HIGHLY REGENERABLE SORBENT
US2011286902
[ PDF ]
A process for the efficient capture of CO2 and sulfur from combustion flue gas streams and gasification based fuel gas mixtures using regenerable and recyclable calcium based sorbents. The regeneration of the calcium sorbent is accomplished by hydrating the sorbent at high temperatures of about 600 DEG C. and a pressure higher than 6 bars to lower the parasitic energy consumption.
BACKGROUND AND SUMMARY OF THE INVENTION
[0003] The concept of utilizing lime for carbon dioxide capture has existed for well over a century. It was first introduced by DuMotay and Marechal in 1869 for enhancing the gasification of coal using lime followed by CONSOL's CO2 acceptor process a century later when this concept was tested in a 40 tons/day plant. A variation of this process called the Hypring process was developed in Japan for the production of hydrogen at high pressures. Harrison et al. and Grace et al. have also applied this concept to the production of hydrogen both from Syngas by the water gas shift reaction and methane by the sorption enhanced steam methane reforming reaction. Silaban et al. studied the reversibility of the carbonation reaction for the production of hydrogen.
[0004] Within the last decade research has also focused on the use of lime for carbon dioxide capture from combustion flue gas. Shimizu et al. conceptually designed a process that uses twin-fluidized bed reactors for capturing carbon dioxide from a coal combustion power plant. After the conceptual design, a significant amount of research has advanced the concept greatly. The contribution of John R. Grace from the University of British Columbia, Juan Carlos Abanades from Instituto Nacional del Carbon-CSIC and CANMET energy Technology Centre have further enhanced the understanding of the Chemical Looping Technology using lime sorbent for the capture of CO2. In addition, the reversibility of the carbonation reaction, the investigation of the decay of CO2 capture over multiple cycles of carbonation and calcination and the production layer formation have been studied by Barker et al., Bhatia and Perlmutter and Mess et al. respectively.
[0005] The regenerability of the calcium oxide sorbent has been the major draw back of high temperature calcium based CO2 capture processes. CaO oxide sorbents are prone to sintering during to the regeneration step which is conducted at high temperatures. Over multiple cycles sintering progressively increases and reduces the CO2 capture capacity of the sorbent. Sintering results in an increase in solid circulation and make up rate. Research has been conducted to develop methods of reducing the sintering of the sorbent. Pretreatment methods have been developed at the CANMET Energy Center which involves hydration of the calcined sorbent at 100[deg.] C. at atmospheric pressure and saturation pressure, powdering the sorbent and preheating the sorbent in a nitrogen atmosphere. The sintering of the sorbent was reduced when these pretreatment methods were applied to the sorbent. This concept developed by CANMET Energy Center is only a pretreatment method and is applied to the sorbent once in 20 cycles and sorbent sintering still occurs resulting in a reduction in CO2 capture capacity. This concept has been tested by Manovic et al. in TGA, fixed bed and a 75 KWth dual fluidized bed combustion plant.
[0006] Grace et al. have also investigated the pretreatment of the sorbent by hydration at atmospheric pressure at 150[deg.] C. and 300[deg.] C. From thermodynamics it is seen that complete hydration does not occur spontaneously at temperature of 300[deg.] C. and hence complete reactivation of the sorbent is not achieved by these methods. In addition, this method has also been developed to be applied once in a few cycles and hence sorbent degradation still occurs.
[0007] The reactivation of the sorbent by recarbonation has also been investigated but this process requires an additional calcination step which is very energy consuming and uneconomical.
[0008] Zeeman et al. have integrated the hydration process as a reactivation step in the CO2 removal process. They hydrate the sorbent at 300[deg.] C. in the presence of CO2 and steam at atmospheric pressure. There has been no mention about the extent of hydration achieved by this process and the amount of carbonation occurring during the hydration process. Although this method was found to reduce sintering and reactivate the sorbent a steady decline in the reactivity of the sorbent was still observed.
[0009] Consequently, it can be understood that there is a need for a cost effective and efficient system and method to minimize the sintering of the selected sorbent and overcome the sorbents decay in reactivity. Exemplary systems and methods of the inventive concept satisfy these needs/preferences.
SUMMARY OF THE INVENTIVE CONCEPT
[0010] Exemplary embodiments of the present invention are directed to economically feasible options for the integration of calcium sorbent based CO2 capture technology in post-combustion systems, specifically in coal-fired power plants. Exemplary embodiments described herein may also be applicable for pre-combustion systems. Exemplary embodiments of the inventive concept specifically provide process integration options while factoring in such variables as location(s) of flue/fuel gas drawn for CO2 capture, source of steam for the hydrator, solids purge and recycle locations, particle capture devices (PCDs), reactor configurations, heat management, and a variety of other factors.
[0011] In contrast to the above mentioned methods of sorbent reactivation. The Ohio State University has developed a process to completely reactivate the sorbent in an energy efficient manner using pressure hydration. The complete reactivation of the sorbent during every cycle reverses the effect of sintering and the history of the number of cycles is completely lost. Hence, this process minimizes the amount of solids circulation in the system. In addition, pressure hydration of the sorbent may be conducted at high temperatures of 600[deg.] C. and the exothermic energy of hydration is used to supply the endothermic energy of dehydration. In addition, pressure hydration does not require the cooling and reheating of the sorbent thereby reducing the parasitic energy consumption of the process. Extensive experiments have been conducted at the Ohio State University wherein complete regeneration of the sorbent has been observed for a number of cycles. Pressure hydration as used herein does not require saturation pressure or high pressure of operation. A pressure of above 6 bar is sufficient for a temperature of 600[deg.] C. As the temperature decreases the pressure required is also reduced. Thus, the hydration process proposed by the Ohio state University is energy efficient and economical.
[0012] Embodiments of the present invention detail a process for the efficient capture of CO2 and sulfur from combustion flue gas streams and gasification based fuel gas mixtures using regenerable and recyclable calcium based sorbents. In exemplary embodiments, the solid sorbent is predominantly a metal oxide that can be converted into a hydrate. Some exemplary embodiments specifically provide a method of reactivating the sorbent by hydrating it at a high temperature of about 600[deg.] C. and a pressure higher than about 6 bars in order to lower the parasitic energy consumption of the process. In other exemplary embodiments, hydration occurs at temperatures high enough such that heat generated from exothermic reaction can be extracted to generate steam for a steam turbine or used for heat exchange; minimum of at least 300[deg.] C. and greater for steam turbine integration. At higher hydration temperatures, greater than about 500[deg.] C., process efficiency increases, but hydration must operate at pressures greater than 1 atm. At temperatures between about 300[deg.] C. to about 500[deg.] C. hydration may occur at about 1 atmosphere. More specifically, temperature from between 350[deg.] C. and about 512[deg.] C. By hydrating the sorbent at high temperatures the energy loss due to solids heating and cooling can be avoided and most crucially the exothermic energy of hydration can be used to provide the energy required for the dehydration of the sorbent or to generate high quality steam for additional electricity generation. At high temperatures of 600[deg.] C., the hydration reaction proceeds to completion only at pressures higher than 6 bars and hence the hydration is conducted at high pressures.
[0013] In other exemplary embodiments at different temperatures of sorbent hydration, the pressure must also be adjusted to maintain maximum reactivity. This reactivation procedure which follows the calcination step during every carbonation calcination cycle produces a high capacity regenerable sorbent which aids in lowering the total amount of solids in circulation making the CO2 capture process economically attractive...
INTEGRATION OF REFORMING/WATER SPLITTING AND ELECTROCHEMICAL SYSTEMS FOR POWER GENERATION WITH INTEGRATED CARBON CAPTURE
WO2011031755
[ PDF ]
High efficiency electricity generation processes and systems with substantially zero CO2 emissions are provided. A closed looping between the unit that generates gaseous fuel (H2, CO, etc) and the fuel cell anode side is formed. In certain embodiments, the heat and exhaust oxygen containing gas from the fuel cell cathode side are also utilized for the gaseous fuel generation. The systems for converting fuel may comprise reactors configured to conduct oxidation- reduction reactions. The resulting power generation efficiencies are improved due to the minimized steam consumption for the gaseous fuel production.in the fuel cell anode loop as well as the strategic mass and energy integration schemes.
The present invention is generally directed to systems and methods of electricity generation with in-situ CO2 capture. In certain embodiments, a reduction- oxidation (redox) system using one or more chemical intermediates is utilized to convert carbonaceous fuel with C([3/4] capture. This is followed by strategic integration with an electrochemical conversion device to produce electricity. In other embodiments, water splitting systems are integrated with the electrochemical systems. Through the process integrations, the process auxiliary power consumption and/or water utilization and energy used for steam generation are minimized.
Fossil fuels including crude oil, natural gas, and coal represent the majority of today's energy supply worldwide. The use of fossil fuels, however, requires that they be transformed to a carrier such as heat, electricity, liquid fuels, or chemicals through chemical conversion processes. With an increasing energy demand and concomitant concerns over the carbon emissions from fossil fuel usage, extensive efforts have been geared toward developing carbon neutral, efficient and economical energy systems that are sustainable. A transition from the use of fossil fuels to that of nuclear and renewable resources such as solar and biomass, thus, represents the natural progression of such efforts. Existing electricity generation technologies have one or more of the following limitations/drawbacks: 1) high costs (e.g., photovoltaic, gasification, ultra- supercritical pulverized coal combustion); 2) low efficiency (e.g., sub-critical pulverized coal combustion); 3) environmental concerns (e.g., fossil fuel power plants); and 4) safety concerns (e.g., nuclear power). One of the common issues with respect to a conventional thermal power plant is the large amount of exergy loss during cooling and reheating of steam. A system and method that minimizes the requirements for steam generation is thus desirable.
Chemical reactions between carbonaceous fuels and air/steam/C02 through the assistance of a reaction medium may represent an effective way to minimize exergy loss in the fuel conversion process. A number of techniques have been proposed to convert carbonaceous fuels using metal oxide. For example, Watkins, U.S. Patent No. 3,027,238, describes a method for producing hydrogen gas including reducing a metal oxide in a reducing zone, and oxidizing the reduced metal with steam to produce hydrogen in an oxidizing zone. This technique, however, is limited to gaseous fuel conversion. Moreover, the gaseous fuel is only partially converted by the metal oxide. Thomas, US Patent No. 7,767,191; Fan, PCT Application No. WO 2007082089; and Fan, PCT Application No. WO 2010037011 describe methods for producing hydrogen gas by reducing a metal oxide in a reduction reaction between a carbon-based fuel and a metal oxide to provide a reduced metal or metal oxide having a lower oxidation state, and oxidizing the reduced metal or metal oxide to produce hydrogen and a metal oxide having a higher oxidation state.
Hydrogen can also be produced from water splitting through photoelectrolysis, thermolysis, and thermochemical routes. To produce electricity, the aforementioned processes teach the further conversion of the hydrogen product in a gas turbine, gas engine, and/or fuel cell. However, a large amount of steam is used in these processes for hydrogen generation. Simple conversion of hydrogen in conventional hydrogen fueled power generation devices will lead to cooling and reheating of large amounts of steam/water, resulting in a large irreversibility of the power generation system.
With increasing demand for electricity, the need arises for improved processes, systems, and system components therein, which produce electricity with higher efficiency and fewer pollutants.
Embodiments of the present invention are generally directed to high efficiency electricity generation processes and systems with substantially zero CO2 emissions. A closed loop between the unit that generates gaseous fuel (H2, CO, etc.) and the fuel cell anode side is formed. In certain embodiments, the heat and exhaust oxygen containing gas from the fuel cell cathode side are also utilized for the gaseous fuel generation. The power generation efficiencies of the systems disclosed herein are significantly greater than state-of-the-art approaches due to the minimized steam consumption for the gaseous fuel production, in the fuel cell anode loop, as well as the strategic mass and energy integration schemes...
Novel redox based systems for fuel and chemical production with in- situ CO2 capture are provided. A redox system using one or more chemical intermediates is utilized in conjunction with liquid fuel generation via indirect Fischer-Tropsch synthesis, direct hydro genation, or pyrolysis. The redox system is used to generate a hydrogen rich stream and/or CO2 and/or heat for liquid fuel and chemical production. A portion of the byproduct fuels and/or steam from liquid fuel and chemical synthesis is used as part of the feedstock for the redox system
The present invention is generally directed to systems and methods for synthetic fuels and chemical products generation with in-situ C02 capture. A reduction- oxidation (redox) system using one or more chemical intermediates is generally utilized in conjunction with liquid fuel generation via indirect C02 hydrogenation, direct hydrogenation, or pyrolysis.
Fossil fuels including crude oil, natural gas, and coal provide more than 85% of today's energy supply. These fossil fuels are usually transformed to carriers such as electricity and liquid transportation fuels prior to utilization by end consumers. Electricity is mainly produced by relatively abundant energy sources such as coal, natural gas, and nuclear. In contrast, liquid transportation fuel is almost exclusively obtained from crude oil, whose supply is relatively insecure with volatile prices. With an increasing energy demand and concomitant concerns over carbon emissions from fossil fuel usage, affordable synthetic transportation fuels from more abundant resources such as coal, biomass, and oil shale are desirable. To address the environmental concerns, the next generation synthetic fuel production processes need to be able to capture pollutants generated in the process. These pollutants include C02, sulfur compounds, and mercury, among others.
Synthetic fuel is generated from gaseous fuels such as natural gas through reforming and the Fischer- Tropsch ("F-T") scheme. Solid fuels such as coal, biomass, and pet coke can be converted to synthetic fuel through indirect liquefaction (gasification - water gas shift - Fischer- Tropsch), direct liquefaction, or pyrolysis. These systems are, however, more capital intensive than oil refining processes. Moreover, their energy conversion efficiencies are relatively low.
Synthetic fuel can also be generated from biomass via biochemical routes.
However, a large amount of process water is utilized. Moreover, the biochemical approaches have stringent requirements on the feedstock.
All the aforementioned processes involve C02 emissions. C02 capture from these processes associates with notable energy losses and hence decreases in process efficiency. Embodiments of the present invention provide alternatives to produce synthetic fuel from naturally occurring carbonaceous fuel sources with high efficiency and effective C02 capture.
Embodiments of the present invention are generally directed to novel redox based systems for fuel and chemical production with in-situ C02 capture. A redox system using one or more chemical intermediates is generally utilized in conjunction with liquid fuel generation via indirect Fischer- Tropsch synthesis, direct hydrogenation, or pyrolysis. The redox system is used to generate a hydrogen rich stream and/or C02 and/or heat for liquid fuel and chemical production. A portion of the byproduct fuels and/or steam from liquid fuel and chemical synthesis is used as part of the feedstock for the redox system.
SYSTEMS FOR CONVERTING FUEL
WO2012155054
[ PDF ]
Technical Background
There is a constant need for clean and efficient energy generation systems. Most of the commercial processes that generate energy carriers such as steam, hydrogen, synthesis gas (syngas), liquid fuels and/or electricity are based on fossil fuels. Furthermore, the dependence on fossil fuels is expected to continue in the foreseeable future due to the much lower costs compared to renewable sources. Currently, the conversion of carbonaceous fuels such as coal, natural gas, petroleum coke is usually conducted through a combustion or reforming process. However, combustion of carbonaceous fuels, especially coal, is a carbon intensive process that emits large quantities of carbon dioxide to the environment. Sulfur and nitrogen compounds are also generated in this process due to the complex content in coal.
Chemical reactions between metal oxides and carbonaceous fuels, on the other hand, may provide a better way to recover the energy stored in the fuels. Several processes are based on the reaction of metal oxide particles with carbonaceous fuels to produce useful energy carriers. For example, Ishida et al. U.S. Pat. No. 5,447,024 describes processes wherein nickel oxide particles are used to convert natural gas through a chemical looping process into heat, which may be used in a turbine. However, recyclability of pure metal oxides is poor and constitutes an impediment for its use in commercial and industrial processes. Moreover, this technology has limited applicability, because it may only convert natural gas, which is more costly than other fossil fuels. Another well known process is a steam-iron process, wherein coal derived producer gas is reacted with iron oxide particles in a fhiidized bed reactor to be later regenerated with steam to produce hydrogen gas. This process however suffers from poor gas conversion rates due to improper contact between reacting solids and gases, and is incapable of producing a hydrogen rich stream.
As demands increase for cleaner and more efficient systems of converting fuel, the need arises for improved systems, and system components therein, which will convert fuel effectively, while reducing pollutants.
The concepts of the present disclosure are generally applicable to systems for producing hydrogen from coal, or other carbonaceous fuels. In accordance with one embodiment of the present disclosure, a system for converting fuel may comprise a first moving bed reactor, a second reactor, and a non-mechanical valve. The first moving bed reactor may comprise at least one tapered section and multiple injection gas ports. The multiple injection gas ports may be configured to deliver a fuel to the first moving bed reactor. The first moving bed reactor may be configured to reduce an oxygen carrying material with a fuel by defining a countercurrent flowpath for the fuel relative to the oxygen carrying material. The second reactor may communicate with the first moving bed reactor and may be operable to receive an oxygen source. The second reactor may be configured to regenerate the reduced oxygen carrying material by oxidation. The non-mechanical valve may comprise a circuitous piping assembly disposed between the first moving bed reactor and the second reactor. At least one gas opening may be configured to receive a gas stream. The gas stream may be operable to reduce gas leakage between the first moving bed reactor and the second reactor...
OXYGEN CARRYING MATERIALS
WO2012155059
[ PDF ]
In accordance with one embodiment of the present disclosure, an oxygen carrying material may include a primary active mass, a primary support material, and a secondary support material. The oxygen carrying material may include about 20% to about 70% by weight of the primary active mass, the primary active mass including a composition having a metal or metal oxide selected from the group consisting of Fe, Co, Ni, Cu, Mo, Mn, Sn, Ru, Rh, and combinations thereof. The oxygen carrying material may include about 5% to about 70% by weight of a primary support material. The oxygen carrying material may include about 1% to about 35% by mass of a secondary support material.
OXYGEN CARRYING MATERIALS
This application claims the benefit of prior-filed U.S. Provisional Patent Application Ser. No. 61/484,982, filed May 11, 2011, the subject matter of which is hereby incorporated by reference in its entirety. The present invention relates to oxygen carrying materials, and specifically to oxygen carrying materials that are associated with chemical looping systems.
There is a constant need for clean and efficient energy generation systems. Most of the commercial processes that generate energy carriers such as steam, hydrogen, synthesis gas (syngas), liquid fuels and/or electricity are based on fossil fuels. Furthermore, the dependence on fossil fuels is expected to continue in the foreseeable future due to the lower costs compared to renewable sources. Currently, the conversion of carbonaceous fuels such as coal, natural gas, and petroleum coke is usually conducted through a combustion or reforming process. However, combustion of carbonaceous fuels, especially coal, is a carbon intensive process that emits large quantities of carbon dioxide to the environment. Sulfur and nitrogen compounds are also generated in this process due to the complex content in coal.
Traditionally the chemical energy stored inside coal has been utilized by combustion with 02, with C02 and H20 as products. Similar reactions can be carried out if instead of oxygen, an oxygen carrying material is used in a chemical looping process. For example, metal oxides such as Fe203 can act as suitable oxygen carrying materials. However, unlike combustion of fuel with air, there is a relatively pure sequestration ready C02 stream produced on combustion with metal oxide carriers. The reduced form of metal oxide may then be reacted with air to liberate heat to produce electricity or reacted with steam to form a relatively pure stream of hydrogen, which can then be used for a variety of purposes.
Chemical reactions between metal oxides and carbonaceous fuels, on the other hand, may provide a better way to recover the energy stored in the fuels. Several processes are based on the reaction of metal oxide particles with carbonaceous fuels to produce useful energy carriers. For example, Ishida et al. (U.S. Pat. No. 5,447,024) describes processes wherein nickel oxide particles are used to convert natural gas through a chemical looping process into heat, which may be used in a turbine. However, recyclability of pure metal oxides is poor and constitutes an impediment for its use in commercial and industrial processes. Moreover, this technology has limited applicability, because it can only convert natural gas, which is more costly than other fossil fuels. Another well known process is a steam-iron process, wherein coal derived producer gas is reacted with iron oxide particles in a fluidized bed reactor to be later regenerated with steam to produce hydrogen gas. This process however suffers from poor gas conversion rates due to improper contact between reacting solids and gases, and is incapable of producing a hydrogen rich stream.
One of the problems with the prior art in combustion looping systems has been the metal/metal oxide oxygen carrying material. For example, iron in the form of small particles may degrade and break up in the reactor. Iron oxide has little mechanical strength as well. After only a few redox cycles, the activity and oxygen carrying capacity of the metal/metal oxide may decline considerably. Replacing the oxygen carrying material with additional fresh metal/metal oxide makes the process more costly.
As demands increase for cleaner and more efficient systems of converting fuel, the need arises for improved systems, and system components therein, which will convert fuel effectively, while reducing pollutants.
The concepts of the present disclosure are generally applicable to oxygen carrying materials. In accordance with one embodiment of the present disclosure, an oxygen carrying material may comprise a primary active mass, a primary support material, and a secondary support material. The oxygen carrying material may comprise about 20% to about 70% by weight of the primary active mass, the primary active mass comprising a composition having a metal or metal oxide selected from the group consisting of Fe, Co, Ni, Cu, Mo, Mn, Sn, Ru, Rh, and combinations thereof. The oxygen carrying material may comprise about 5% to about 70% by weight of a primary support material. The primary support material may comprise a composition having at least one metal, metal oxide, metal carbide, metal nitrate, metal halide, or combinations thereof; at least one ceramic or clay material, or salts thereof; at least one naturally occurring ore; or combinations thereof. The oxygen carrying material may comprise about 1% to about 35% by mass of a secondary support material. The secondary support material may comprise a composition having at least one metal, metal oxide, metal carbide, metal nitrate, metal halide, or combinations thereof; at least one ceramic or clay material or salts thereof; at least one naturally occurring ore; or combinations thereof. The primary support material composition and the secondary support material composition may be different. In accordance with another embodiment of the present disclosure, a system for converting fuel may comprise an oxygen carrying material, a first reactor comprising a moving bed and an inlet for providing fuel to the first reactor, wherein the first reactor is configured to reduce the oxygen carrying material with the fuel to produce a reduced oxygen carrying material, and a second reactor communicating with the first reactor and an oxygen source, wherein the second reactor is configured to regenerate the oxygen carrying material by oxidizing the oxygen carrying material.
In accordance with another embodiment of the present disclosure, a method for synthesizing an oxygen carrying material may include forming a matrix comprising a primary active mass, a primary support, and a secondary support; drying the matrix; and forming the matrix into particles of the oxygen carrying material...
CARBONATION CALCINATION REACTION PROCESS FOR CO2 CAPTURE USING A HIGHLY REGENERABLE SORBENT
US2011286902
[ PDF ]
A process for the efficient capture of CO2 and sulfur from combustion flue gas streams and gasification based fuel gas mixtures using regenerable and recyclable calcium based sorbents. The regeneration of the calcium sorbent is accomplished by hydrating the sorbent at high temperatures of about 600 DEG C. and a pressure higher than 6 bars to lower the parasitic energy consumption.
BACKGROUND AND SUMMARY OF THE INVENTION
[0003] The concept of utilizing lime for carbon dioxide capture has existed for well over a century. It was first introduced by DuMotay and Marechal in 1869 for enhancing the gasification of coal using lime followed by CONSOL's CO2 acceptor process a century later when this concept was tested in a 40 tons/day plant. A variation of this process called the Hypring process was developed in Japan for the production of hydrogen at high pressures. Harrison et al. and Grace et al. have also applied this concept to the production of hydrogen both from Syngas by the water gas shift reaction and methane by the sorption enhanced steam methane reforming reaction. Silaban et al. studied the reversibility of the carbonation reaction for the production of hydrogen.
[0004] Within the last decade research has also focused on the use of lime for carbon dioxide capture from combustion flue gas. Shimizu et al. conceptually designed a process that uses twin-fluidized bed reactors for capturing carbon dioxide from a coal combustion power plant. After the conceptual design, a significant amount of research has advanced the concept greatly. The contribution of John R. Grace from the University of British Columbia, Juan Carlos Abanades from Instituto Nacional del Carbon-CSIC and CANMET energy Technology Centre have further enhanced the understanding of the Chemical Looping Technology using lime sorbent for the capture of CO2. In addition, the reversibility of the carbonation reaction, the investigation of the decay of CO2 capture over multiple cycles of carbonation and calcination and the production layer formation have been studied by Barker et al., Bhatia and Perlmutter and Mess et al. respectively.
[0005] The regenerability of the calcium oxide sorbent has been the major draw back of high temperature calcium based CO2 capture processes. CaO oxide sorbents are prone to sintering during to the regeneration step which is conducted at high temperatures. Over multiple cycles sintering progressively increases and reduces the CO2 capture capacity of the sorbent. Sintering results in an increase in solid circulation and make up rate. Research has been conducted to develop methods of reducing the sintering of the sorbent. Pretreatment methods have been developed at the CANMET Energy Center which involves hydration of the calcined sorbent at 100[deg.] C. at atmospheric pressure and saturation pressure, powdering the sorbent and preheating the sorbent in a nitrogen atmosphere. The sintering of the sorbent was reduced when these pretreatment methods were applied to the sorbent. This concept developed by CANMET Energy Center is only a pretreatment method and is applied to the sorbent once in 20 cycles and sorbent sintering still occurs resulting in a reduction in CO2 capture capacity. This concept has been tested by Manovic et al. in TGA, fixed bed and a 75 KWth dual fluidized bed combustion plant.
[0006] Grace et al. have also investigated the pretreatment of the sorbent by hydration at atmospheric pressure at 150[deg.] C. and 300[deg.] C. From thermodynamics it is seen that complete hydration does not occur spontaneously at temperature of 300[deg.] C. and hence complete reactivation of the sorbent is not achieved by these methods. In addition, this method has also been developed to be applied once in a few cycles and hence sorbent degradation still occurs.
[0007] The reactivation of the sorbent by recarbonation has also been investigated but this process requires an additional calcination step which is very energy consuming and uneconomical.
[0008] Zeeman et al. have integrated the hydration process as a reactivation step in the CO2 removal process. They hydrate the sorbent at 300[deg.] C. in the presence of CO2 and steam at atmospheric pressure. There has been no mention about the extent of hydration achieved by this process and the amount of carbonation occurring during the hydration process. Although this method was found to reduce sintering and reactivate the sorbent a steady decline in the reactivity of the sorbent was still observed.
[0009] Consequently, it can be understood that there is a need for a cost effective and efficient system and method to minimize the sintering of the selected sorbent and overcome the sorbents decay in reactivity. Exemplary systems and methods of the inventive concept satisfy these needs/preferences.
SUMMARY OF THE INVENTIVE CONCEPT
[0010] Exemplary embodiments of the present invention are directed to economically feasible options for the integration of calcium sorbent based CO2 capture technology in post-combustion systems, specifically in coal-fired power plants. Exemplary embodiments described herein may also be applicable for pre-combustion systems. Exemplary embodiments of the inventive concept specifically provide process integration options while factoring in such variables as location(s) of flue/fuel gas drawn for CO2 capture, source of steam for the hydrator, solids purge and recycle locations, particle capture devices (PCDs), reactor configurations, heat management, and a variety of other factors.
[0011] In contrast to the above mentioned methods of sorbent reactivation. The Ohio State University has developed a process to completely reactivate the sorbent in an energy efficient manner using pressure hydration. The complete reactivation of the sorbent during every cycle reverses the effect of sintering and the history of the number of cycles is completely lost. Hence, this process minimizes the amount of solids circulation in the system. In addition, pressure hydration of the sorbent may be conducted at high temperatures of 600[deg.] C. and the exothermic energy of hydration is used to supply the endothermic energy of dehydration. In addition, pressure hydration does not require the cooling and reheating of the sorbent thereby reducing the parasitic energy consumption of the process. Extensive experiments have been conducted at the Ohio State University wherein complete regeneration of the sorbent has been observed for a number of cycles. Pressure hydration as used herein does not require saturation pressure or high pressure of operation. A pressure of above 6 bar is sufficient for a temperature of 600[deg.] C. As the temperature decreases the pressure required is also reduced. Thus, the hydration process proposed by the Ohio state University is energy efficient and economical.
[0012] Embodiments of the present invention detail a process for the efficient capture of CO2 and sulfur from combustion flue gas streams and gasification based fuel gas mixtures using regenerable and recyclable calcium based sorbents. In exemplary embodiments, the solid sorbent is predominantly a metal oxide that can be converted into a hydrate. Some exemplary embodiments specifically provide a method of reactivating the sorbent by hydrating it at a high temperature of about 600[deg.] C. and a pressure higher than about 6 bars in order to lower the parasitic energy consumption of the process. In other exemplary embodiments, hydration occurs at temperatures high enough such that heat generated from exothermic reaction can be extracted to generate steam for a steam turbine or used for heat exchange; minimum of at least 300[deg.] C. and greater for steam turbine integration. At higher hydration temperatures, greater than about 500[deg.] C., process efficiency increases, but hydration must operate at pressures greater than 1 atm. At temperatures between about 300[deg.] C. to about 500[deg.] C. hydration may occur at about 1 atmosphere. More specifically, temperature from between 350[deg.] C. and about 512[deg.] C. By hydrating the sorbent at high temperatures the energy loss due to solids heating and cooling can be avoided and most crucially the exothermic energy of hydration can be used to provide the energy required for the dehydration of the sorbent or to generate high quality steam for additional electricity generation. At high temperatures of 600[deg.] C., the hydration reaction proceeds to completion only at pressures higher than 6 bars and hence the hydration is conducted at high pressures.
[0013] In other exemplary embodiments at different temperatures of sorbent hydration, the pressure must also be adjusted to maintain maximum reactivity. This reactivation procedure which follows the calcination step during every carbonation calcination cycle produces a high capacity regenerable sorbent which aids in lowering the total amount of solids in circulation making the CO2 capture process economically attractive...
INTEGRATION OF REFORMING/WATER SPLITTING AND ELECTROCHEMICAL SYSTEMS FOR POWER GENERATION WITH INTEGRATED CARBON CAPTURE
WO2011031755
[ PDF ]
High efficiency electricity generation processes and systems with substantially zero CO2 emissions are provided. A closed looping between the unit that generates gaseous fuel (H2, CO, etc) and the fuel cell anode side is formed. In certain embodiments, the heat and exhaust oxygen containing gas from the fuel cell cathode side are also utilized for the gaseous fuel generation. The systems for converting fuel may comprise reactors configured to conduct oxidation- reduction reactions. The resulting power generation efficiencies are improved due to the minimized steam consumption for the gaseous fuel production.in the fuel cell anode loop as well as the strategic mass and energy integration schemes.
The present invention is generally directed to systems and methods of electricity generation with in-situ CO2 capture. In certain embodiments, a reduction- oxidation (redox) system using one or more chemical intermediates is utilized to convert carbonaceous fuel with C([3/4] capture. This is followed by strategic integration with an electrochemical conversion device to produce electricity. In other embodiments, water splitting systems are integrated with the electrochemical systems. Through the process integrations, the process auxiliary power consumption and/or water utilization and energy used for steam generation are minimized.
Fossil fuels including crude oil, natural gas, and coal represent the majority of today's energy supply worldwide. The use of fossil fuels, however, requires that they be transformed to a carrier such as heat, electricity, liquid fuels, or chemicals through chemical conversion processes. With an increasing energy demand and concomitant concerns over the carbon emissions from fossil fuel usage, extensive efforts have been geared toward developing carbon neutral, efficient and economical energy systems that are sustainable. A transition from the use of fossil fuels to that of nuclear and renewable resources such as solar and biomass, thus, represents the natural progression of such efforts. Existing electricity generation technologies have one or more of the following limitations/drawbacks: 1) high costs (e.g., photovoltaic, gasification, ultra- supercritical pulverized coal combustion); 2) low efficiency (e.g., sub-critical pulverized coal combustion); 3) environmental concerns (e.g., fossil fuel power plants); and 4) safety concerns (e.g., nuclear power). One of the common issues with respect to a conventional thermal power plant is the large amount of exergy loss during cooling and reheating of steam. A system and method that minimizes the requirements for steam generation is thus desirable.
Chemical reactions between carbonaceous fuels and air/steam/C02 through the assistance of a reaction medium may represent an effective way to minimize exergy loss in the fuel conversion process. A number of techniques have been proposed to convert carbonaceous fuels using metal oxide. For example, Watkins, U.S. Patent No. 3,027,238, describes a method for producing hydrogen gas including reducing a metal oxide in a reducing zone, and oxidizing the reduced metal with steam to produce hydrogen in an oxidizing zone. This technique, however, is limited to gaseous fuel conversion. Moreover, the gaseous fuel is only partially converted by the metal oxide. Thomas, US Patent No. 7,767,191; Fan, PCT Application No. WO 2007082089; and Fan, PCT Application No. WO 2010037011 describe methods for producing hydrogen gas by reducing a metal oxide in a reduction reaction between a carbon-based fuel and a metal oxide to provide a reduced metal or metal oxide having a lower oxidation state, and oxidizing the reduced metal or metal oxide to produce hydrogen and a metal oxide having a higher oxidation state.
Hydrogen can also be produced from water splitting through photoelectrolysis, thermolysis, and thermochemical routes. To produce electricity, the aforementioned processes teach the further conversion of the hydrogen product in a gas turbine, gas engine, and/or fuel cell. However, a large amount of steam is used in these processes for hydrogen generation. Simple conversion of hydrogen in conventional hydrogen fueled power generation devices will lead to cooling and reheating of large amounts of steam/water, resulting in a large irreversibility of the power generation system.
With increasing demand for electricity, the need arises for improved processes, systems, and system components therein, which produce electricity with higher efficiency and fewer pollutants.
Embodiments of the present invention are generally directed to high efficiency electricity generation processes and systems with substantially zero CO2 emissions. A closed loop between the unit that generates gaseous fuel (H2, CO, etc.) and the fuel cell anode side is formed. In certain embodiments, the heat and exhaust oxygen containing gas from the fuel cell cathode side are also utilized for the gaseous fuel generation. The power generation efficiencies of the systems disclosed herein are significantly greater than state-of-the-art approaches due to the minimized steam consumption for the gaseous fuel production, in the fuel cell anode loop, as well as the strategic mass and energy integration schemes...
SYNTHETIC FUELS AND CHEMICALS PRODUCTION WITH IN-SITU CO2 CAPTURE
WO2011031752
Novel redox based systems for fuel and chemical production with in- situ CO2 capture are provided. A redox system using one or more chemical intermediates is utilized in conjunction with liquid fuel generation via indirect Fischer-Tropsch synthesis, direct hydro genation, or pyrolysis. The redox system is used to generate a hydrogen rich stream and/or CO2 and/or heat for liquid fuel and chemical production. A portion of the byproduct fuels and/or steam from liquid fuel and chemical synthesis is used as part of the feedstock for the redox system
The present invention is generally directed to systems and methods for synthetic fuels and chemical products generation with in-situ C02 capture. A reduction- oxidation (redox) system using one or more chemical intermediates is generally utilized in conjunction with liquid fuel generation via indirect C02 hydrogenation, direct hydrogenation, or pyrolysis.
Fossil fuels including crude oil, natural gas, and coal provide more than 85% of today's energy supply. These fossil fuels are usually transformed to carriers such as electricity and liquid transportation fuels prior to utilization by end consumers. Electricity is mainly produced by relatively abundant energy sources such as coal, natural gas, and nuclear. In contrast, liquid transportation fuel is almost exclusively obtained from crude oil, whose supply is relatively insecure with volatile prices. With an increasing energy demand and concomitant concerns over carbon emissions from fossil fuel usage, affordable synthetic transportation fuels from more abundant resources such as coal, biomass, and oil shale are desirable. To address the environmental concerns, the next generation synthetic fuel production processes need to be able to capture pollutants generated in the process. These pollutants include C02, sulfur compounds, and mercury, among others.
Synthetic fuel is generated from gaseous fuels such as natural gas through reforming and the Fischer- Tropsch ("F-T") scheme. Solid fuels such as coal, biomass, and pet coke can be converted to synthetic fuel through indirect liquefaction (gasification - water gas shift - Fischer- Tropsch), direct liquefaction, or pyrolysis. These systems are, however, more capital intensive than oil refining processes. Moreover, their energy conversion efficiencies are relatively low.
Synthetic fuel can also be generated from biomass via biochemical routes.
However, a large amount of process water is utilized. Moreover, the biochemical approaches have stringent requirements on the feedstock.
All the aforementioned processes involve C02 emissions. C02 capture from these processes associates with notable energy losses and hence decreases in process efficiency. Embodiments of the present invention provide alternatives to produce synthetic fuel from naturally occurring carbonaceous fuel sources with high efficiency and effective C02 capture.
Embodiments of the present invention are generally directed to novel redox based systems for fuel and chemical production with in-situ C02 capture. A redox system using one or more chemical intermediates is generally utilized in conjunction with liquid fuel generation via indirect Fischer- Tropsch synthesis, direct hydrogenation, or pyrolysis. The redox system is used to generate a hydrogen rich stream and/or C02 and/or heat for liquid fuel and chemical production. A portion of the byproduct fuels and/or steam from liquid fuel and chemical synthesis is used as part of the feedstock for the redox system...
SYSTEMS FOR CONVERTING FUEL
WO2012155054
[ PDF ]Technical Background
There is a constant need for clean and efficient energy generation systems. Most of the commercial processes that generate energy carriers such as steam, hydrogen, synthesis gas (syngas), liquid fuels and/or electricity are based on fossil fuels. Furthermore, the dependence on fossil fuels is expected to continue in the foreseeable future due to the much lower costs compared to renewable sources. Currently, the conversion of carbonaceous fuels such as coal, natural gas, petroleum coke is usually conducted through a combustion or reforming process. However, combustion of carbonaceous fuels, especially coal, is a carbon intensive process that emits large quantities of carbon dioxide to the environment. Sulfur and nitrogen compounds are also generated in this process due to the complex content in coal.
Chemical reactions between metal oxides and carbonaceous fuels, on the other hand, may provide a better way to recover the energy stored in the fuels. Several processes are based on the reaction of metal oxide particles with carbonaceous fuels to produce useful energy carriers. For example, Ishida et al. U.S. Pat. No. 5,447,024 describes processes wherein nickel oxide particles are used to convert natural gas through a chemical looping process into heat, which may be used in a turbine. However, recyclability of pure metal oxides is poor and constitutes an impediment for its use in commercial and industrial processes. Moreover, this technology has limited applicability, because it may only convert natural gas, which is more costly than other fossil fuels. Another well known process is a steam-iron process, wherein coal derived producer gas is reacted with iron oxide particles in a fhiidized bed reactor to be later regenerated with steam to produce hydrogen gas. This process however suffers from poor gas conversion rates due to improper contact between reacting solids and gases, and is incapable of producing a hydrogen rich stream.
As demands increase for cleaner and more efficient systems of converting fuel, the need arises for improved systems, and system components therein, which will convert fuel effectively, while reducing pollutants.
The concepts of the present disclosure are generally applicable to systems for producing hydrogen from coal, or other carbonaceous fuels. In accordance with one embodiment of the present disclosure, a system for converting fuel may comprise a first moving bed reactor, a second reactor, and a non-mechanical valve. The first moving bed reactor may comprise at least one tapered section and multiple injection gas ports. The multiple injection gas ports may be configured to deliver a fuel to the first moving bed reactor. The first moving bed reactor may be configured to reduce an oxygen carrying material with a fuel by defining a countercurrent flowpath for the fuel relative to the oxygen carrying material. The second reactor may communicate with the first moving bed reactor and may be operable to receive an oxygen source. The second reactor may be configured to regenerate the reduced oxygen carrying material by oxidation. The non-mechanical valve may comprise a circuitous piping assembly disposed between the first moving bed reactor and the second reactor. At least one gas opening may be configured to receive a gas stream. The gas stream may be operable to reduce gas leakage between the first moving bed reactor and the second reactor...
BACKGROUND AND SUMMARY OF THE INVENTION
[0003] The concept of utilizing lime for carbon dioxide capture has existed for well over a century. It was first introduced by DuMotay and Marechal in 1869 for enhancing the gasification of coal using lime followed by CONSOL's CO2 acceptor process a century later when this concept was tested in a 40 tons/day plant. A variation of this process called the Hypring process was developed in Japan for the production of hydrogen at high pressures. Harrison et al. and Grace et al. have also applied this concept to the production of hydrogen both from Syngas by the water gas shift reaction and methane by the sorption enhanced steam methane reforming reaction. Silaban et al. studied the reversibility of the carbonation reaction for the production of hydrogen.
[0004] Within the last decade research has also focused on the use of lime for carbon dioxide capture from combustion flue gas. Shimizu et al. conceptually designed a process that uses twin-fluidized bed reactors for capturing carbon dioxide from a coal combustion power plant. After the conceptual design, a significant amount of research has advanced the concept greatly. The contribution of John R. Grace from the University of British Columbia, Juan Carlos Abanades from Instituto Nacional del Carbon-CSIC and CANMET energy Technology Centre have further enhanced the understanding of the Chemical Looping Technology using lime sorbent for the capture of CO2. In addition, the reversibility of the carbonation reaction, the investigation of the decay of CO2 capture over multiple cycles of carbonation and calcination and the production layer formation have been studied by Barker et al., Bhatia and Perlmutter and Mess et al. respectively.
[0005] The regenerability of the calcium oxide sorbent has been the major draw back of high temperature calcium based CO2 capture processes. CaO oxide sorbents are prone to sintering during to the regeneration step which is conducted at high temperatures. Over multiple cycles sintering progressively increases and reduces the CO2 capture capacity of the sorbent. Sintering results in an increase in solid circulation and make up rate. Research has been conducted to develop methods of reducing the sintering of the sorbent. Pretreatment methods have been developed at the CANMET Energy Center which involves hydration of the calcined sorbent at 100[deg.] C. at atmospheric pressure and saturation pressure, powdering the sorbent and preheating the sorbent in a nitrogen atmosphere. The sintering of the sorbent was reduced when these pretreatment methods were applied to the sorbent. This concept developed by CANMET Energy Center is only a pretreatment method and is applied to the sorbent once in 20 cycles and sorbent sintering still occurs resulting in a reduction in CO2 capture capacity. This concept has been tested by Manovic et al. in TGA, fixed bed and a 75 KWth dual fluidized bed combustion plant.
[0006] Grace et al. have also investigated the pretreatment of the sorbent by hydration at atmospheric pressure at 150[deg.] C. and 300[deg.] C. From thermodynamics it is seen that complete hydration does not occur spontaneously at temperature of 300[deg.] C. and hence complete reactivation of the sorbent is not achieved by these methods. In addition, this method has also been developed to be applied once in a few cycles and hence sorbent degradation still occurs.
[0007] The reactivation of the sorbent by recarbonation has also been investigated but this process requires an additional calcination step which is very energy consuming and uneconomical.
[0008] Zeeman et al. have integrated the hydration process as a reactivation step in the CO2 removal process. They hydrate the sorbent at 300[deg.] C. in the presence of CO2 and steam at atmospheric pressure. There has been no mention about the extent of hydration achieved by this process and the amount of carbonation occurring during the hydration process. Although this method was found to reduce sintering and reactivate the sorbent a steady decline in the reactivity of the sorbent was still observed.
[0009] Consequently, it can be understood that there is a need for a cost effective and efficient system and method to minimize the sintering of the selected sorbent and overcome the sorbents decay in reactivity. Exemplary systems and methods of the inventive concept satisfy these needs/preferences.
SUMMARY OF THE INVENTIVE CONCEPT
[0010] Exemplary embodiments of the present invention are directed to economically feasible options for the integration of calcium sorbent based CO2 capture technology in post-combustion systems, specifically in coal-fired power plants. Exemplary embodiments described herein may also be applicable for pre-combustion systems. Exemplary embodiments of the inventive concept specifically provide process integration options while factoring in such variables as location(s) of flue/fuel gas drawn for CO2 capture, source of steam for the hydrator, solids purge and recycle locations, particle capture devices (PCDs), reactor configurations, heat management, and a variety of other factors.
[0011] In contrast to the above mentioned methods of sorbent reactivation. The Ohio State University has developed a process to completely reactivate the sorbent in an energy efficient manner using pressure hydration. The complete reactivation of the sorbent during every cycle reverses the effect of sintering and the history of the number of cycles is completely lost. Hence, this process minimizes the amount of solids circulation in the system. In addition, pressure hydration of the sorbent may be conducted at high temperatures of 600[deg.] C. and the exothermic energy of hydration is used to supply the endothermic energy of dehydration. In addition, pressure hydration does not require the cooling and reheating of the sorbent thereby reducing the parasitic energy consumption of the process. Extensive experiments have been conducted at the Ohio State University wherein complete regeneration of the sorbent has been observed for a number of cycles. Pressure hydration as used herein does not require saturation pressure or high pressure of operation. A pressure of above 6 bar is sufficient for a temperature of 600[deg.] C. As the temperature decreases the pressure required is also reduced. Thus, the hydration process proposed by the Ohio state University is energy efficient and economical.
[0012] Embodiments of the present invention detail a process for the efficient capture of CO2 and sulfur from combustion flue gas streams and gasification based fuel gas mixtures using regenerable and recyclable calcium based sorbents. In exemplary embodiments, the solid sorbent is predominantly a metal oxide that can be converted into a hydrate. Some exemplary embodiments specifically provide a method of reactivating the sorbent by hydrating it at a high temperature of about 600[deg.] C. and a pressure higher than about 6 bars in order to lower the parasitic energy consumption of the process. In other exemplary embodiments, hydration occurs at temperatures high enough such that heat generated from exothermic reaction can be extracted to generate steam for a steam turbine or used for heat exchange; minimum of at least 300[deg.] C. and greater for steam turbine integration. At higher hydration temperatures, greater than about 500[deg.] C., process efficiency increases, but hydration must operate at pressures greater than 1 atm. At temperatures between about 300[deg.] C. to about 500[deg.] C. hydration may occur at about 1 atmosphere. More specifically, temperature from between 350[deg.] C. and about 512[deg.] C. By hydrating the sorbent at high temperatures the energy loss due to solids heating and cooling can be avoided and most crucially the exothermic energy of hydration can be used to provide the energy required for the dehydration of the sorbent or to generate high quality steam for additional electricity generation. At high temperatures of 600[deg.] C., the hydration reaction proceeds to completion only at pressures higher than 6 bars and hence the hydration is conducted at high pressures.
[0013] In other exemplary embodiments at different temperatures of sorbent hydration, the pressure must also be adjusted to maintain maximum reactivity. This reactivation procedure which follows the calcination step during every carbonation calcination cycle produces a high capacity regenerable sorbent which aids in lowering the total amount of solids in circulation making the CO2 capture process economically attractive...
CIRCULATING FLUIDIZED BED WITH MOVING BED DOWNCOMERS AND GAS SEALING BETWEEN REACTORSA system and process for carrying out one or more chemical reactions are provided and include one or more chemical reactors having particulate solids forming a bed therein, and a gas stripping zone forming a non-mechanical seal between said reactors which includes a conduit connecting the reactors. The conduit includes an inlet for a stripping gas which is adapted to prevent process gas from passing between reactors while permitting particulate solids to pass between reactors.
WO2012064712
[ PDF ]