
rexresearch.com
Tamara Sahno / Victor Kurashov
Biological Transmutations
Biological Transmutations
YouTube -- Press Conference of transmutation (Switzerland)
bt-isotopes.com -- Biochemical method of chemical elements transmutation and chemical elements isotopes transformation
RU2563511 -- MICROBIOLOGICAL METHOD OF TRANSMUTATION OF CHEMICAL ELEMENTS AND CONVERSION OF ISOTOPES OF CHEMICAL ELEMENTS
BIOTECHNOLOGICAL METHOD FOR ARTIFICIAL PRODUCING OF ACTINIDES, OTHER VALUABLE RADIOACTIVE ELEMENTS, THEIR ISOTOPES, AND STABLE ISOTOPES OF NOBLE METALS - PLATINUM AND GOLD
ARTIFICIAL OBTAINING OF f-ELEMENTS – ACTINIDES AND OTHER VALUABLE RADIOACTIVE ELEMENTS AND THEIR ISOTOPES, AS WELL AS STABLE ISOTOPES OF PLATINUM AND GOLD WITH THE USE OF MICROORGANISMS
TRANSMUTATION OF CHEMICAL ELEMENTS AND ISOTOPE TRANSFORMATION WITH THE USE OF BIOTECHNOLOGY
RU2052223 -- METHOD FOR PRODUCING STABLE ISOTOPES DUE TO NUCLEAR TRANSMUTATION, SUCH AS LOW-TEMPERATURE NUCLEAR FUSION OF ELEMENTS IN MICROBIOLOGICAL CULTURES
Thiobacillus ferrooxidans
https://www.youtube.com/watch?v=MN0LjXT323s
Press Conference of transmutation
(Switzerland)
Press conference that was held at the Swiss Press Club in Geneva on June 21, 2016 by a Russian Corporate Partnership called Actinedes consisting of inventors Viktor Kurashov and Tamara Sakhno, and the administrator Vladislav Karabanov.
June 21, 2016 in the Swiss capital of Geneva, held a press conference on the epoch-making discovery of the chemical elements transmute biochemically.
The Conference was attended by Tamara Sahno Victor Kurashov - Scientists have made a discovery and Vladislav Karabanov administrator and head of the project.
Transcript :
Vladislav Karabanov: “Today, here in Geneva, we are making public a discovery and a technology which without any exaggeration could be of historic significance.
The essence of this discovery and the technology boils down to the development of an industrial method for the transformation of chemical elements into other elements and their isotopes.
What we’ll have to show you today is the transmutation without nuclear reactors, without heavy water, or anything of the kind, to obtain a transmutation of elements. Our approach to transmutation of chemical elements is biochemical in nature.
It is still too early to fully grasp the economic and civilization significance of this technology. It would not be an exaggeration to say that this discovery is a veritable revolution that’s going to open a new chapter in our technological progress. Unlikely as it may sound, this is a fact.
The architects of this discovery and technology are leading Russian Chemists, Mrs. Tamara Sahno and Mr. Victor Kurashov. These are theoretical and experimental scientists who stand on the shoulders of a dynasty of researchers who have been instrumental in discovering these methods for the transformation of chemical methods.
Mankind, represented by the authors, has discovered this method for the transmutation of matter which is likely to change the face of today’s world, perhaps as deeply as it was changed by the use of electricity, perhaps even deeper.
The repercussions of this revolution will be felt in the energy sector, medicine, industry and perhaps would also open up new industries, brand new industries that will have enormous humanitarian implications.
What is most important to bear in mind is that what we are talking about here is a ready-made industrial approach that will be capable of producing target products in industrial quantities in a matter of months. With respect to the economic aspects of this discovery I am going to brief you about that later . . .”
Victor Kurashov: “Ladies and gentlemen, our work to develop the technology for the transmutation of chemical elements goes back to the early 90s. The very first results were obtained back in 1998, but the bulk of this effort and research, as well as hundreds of successful experiments fall on the Summer and Autumn of 2013.
Our further efforts involved patenting this work, and so for all these reasons we haven’t rushed to publish our findings until the patent was issued. We received the patent priority on the 15th of May 2014, whereas the patent itself was issued on the 25th of August 2015.
Let’s move onto the process itself very briefly. The first component used in the process is ore, or nuclear waste. The second component of the process are valuable valency metals such as vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc, and others. Either of these will do, but we tend to use iron as the least costly element. The third component and a factor in this process, these are bacteria. Usually we use iron and sulphur-reducing bacterial species which we select along a certain list of criteria, such as that the bacteria are active, that they are resistant to radiation, that they are adapted to a heavily salted solution — ore, suspended in water.
Now about the technology itself: ore, or nuclear waste (there’s no difference) is processed by bacteria in the presence of valuable valency elements in any closed vessel. The transmutation process kicks off immediately, and proceed stage by stage for two or three weeks until target elements are obtained. But if it is not stopped on time, this process would carry on until stable isotopes are obtained as the end product.”
The Russian patent RU 2563511 awarded to Mrs. Tamara Sahno and Mr. Victor Kurashov available at Google Patent repository says [in Google translation]:
“The invention relates to the field of biotechnology and chemical transmutation. Radioactive feedstock containing radioactive chemical elements or isotopes, treated with an aqueous suspension of bacteria of the genus Thiobacillus, in the presence of variable valence elements. As the use of radioactive materials or ore radioactive waste nuclear fuel cycle. The process leads to obtaining polonium, radon, France, radium, actinium, thorium, protactinium, uranium, neptunium, americium, nickel, manganese, bromine, hafnium, ytterbium, mercury, gold, platinum, and their isotopes. The invention allows to obtain valuable radioactive elements, to carry out the inactivation of nuclear waste from the conversion of waste radioactive isotopes of elements into stable isotopes. 2 ZP f-ly, 18 ill., 5 tab., 9 pr.
The invention relates to chemical transmutation of radioactive isotopes and transformation, that is to artificially produce some chemical elements from other elements. In particular, the method allows to obtain rare and valuable elements: polonium, radon, francium, radium, and actinides – actinium, thorium, protactinium, uranium, neptunium, and various isotopes of these and other elements.
Known transformation of chemical elements, the formation of new isotopes of elements and new chemical elements during nuclear fission and synthesis of chemical elements used in conventional nuclear rectors, in nuclear power plants (NPPs) in research nuclear reactors, for example, by irradiation of the chemical elements with neutrons or protons, or alpha particles.
A method of obtaining the radionuclide nickel-63 in the reactor from a target comprising obtaining enriched Nickel-62 nickel target, the irradiation target in the reactor, followed by enrichment of irradiated product from nickel-63 at extraction of nickel-64 isotope product (RU 2313149, 2007). The advantage of the method is to obtain a high quality product which is designed for use in stand-alone sources of electrical energy, in the detectors of explosives and so on. The reproducibility of the results was confirmed by the analysis of the isotopic composition of elements by mass spectrometry.
However, the method is complicated and unsafe degree requires industrial safety.
It is also known the transmutation of elements – long-lived radioactive nuclides, including those arising in irradiated nuclear fuel (RU 2415486, 2011). The method consists in irradiating neutron flux transmutable material, the irradiation is carried out with neutrons obtained in the nuclear fusion reactions in the pre-formed neutron source plasma, at a certain placement of the scattering medium neutrons. This method is based on the reactions of nuclear fusion in a tokamak is also complex and requires special equipment.
A method of obtaining radionuclides Th-228 and Ra-224, which is also implemented in a reactor technology. The technology is quite complex and has a safety limit (RU 2317607, 2008).
Thus, upon receipt of the chemical elements and their isotopes, in general, are conventionally used nuclear reactions involving nuclear reactors or other sophisticated equipment at high energy costs.
Attempts have been made to solve the problem of obtaining radioactive isotopes in the process of nuclear transmutation of elements more secure manner using the microorganisms. Known in particular isotopes conversion method using microorganisms comprising growing microbial culture Deinococcus radiodurans on a nutrient medium containing the necessary for transmutation of initial isotopic components, and deficient close chemical analogues of the target element. The composition of the medium is introduced, such starting isotopic components which are radioactive and transmutation process can lead to the formation of the target chemical element in the form of a stable or radioactive isotope, which is absorbed by the microbial culture and then remains steady or remains radioactive or decomposed to the desired stable isotope (RU 2002101281 A, 2003). This method does not provide a high yield of the desired isotope, and also requires the use of ionizing radiation as a trigger and response factor supports.
Also known process for the preparation of stable isotopes by nuclear transmutation type of cold fusion elements in microbial cultures (RU 2052223, 1996). The method consists in the fact that the cells of microorganisms are grown in a culture medium deficient isotope target (target isotopes) impact factors contributing to the destruction of the interatomic bonds and leading to an increase in its concentration of free atoms or ions of hydrogen isotopes. The medium is prepared on the basis of heavy water and injected into it scarce for the environment unstable isotopes that decay at the end to form the desired stable isotopes. As a factor that destroys the interatomic bonds using ionizing radiation. This method is based on the use of ionizing radiation, it is not designed for commercial scale requires a high energy and cost.
All of the chemical elements and their isotopes and by-products obtained until now complex and unsafe traditional methods by conventional nuclear reactions in small (sometimes – in micro) amounts clearly insufficient for the energy, industrial, industrial, technical and scientific needs of mankind. Described microbial process for the transmutation of chemical elements allows you to receive all of these chemical elements and their isotopes in almost unlimited quantities, simple to perform, safe for workers and the public, environmentally friendly way that does not require large material flow rates, heat, electricity and heating, while providing this energy, industrial, technical and scientific problems of civilization. These elements and isotopes are enormous reserves of energy, have an extremely high value and selling price on the market.
Microbiological method is proposed transmutation of the chemical elements and isotopes of chemical conversion elements, characterized in that the radioactive feedstock containing radioactive chemical elements or isotopes, treated with an aqueous suspension of bacteria of the genus Thiobacillus, in the presence of any s, p, d, f-elements with variable valency. Selection of elements with variable valence based on the principle of creating a high redox potential. That is, this selection key, or simply on the orientation of these or other elements of variable valency brought into the reaction medium, a redox potential value which is optimal in the range of 400-800 mV (for example, in Examples 1, 2, 3, 4 Eh = 635 mV, 798 mV, 753 mV and 717 mV, respectively).
Items with variable valence, as in the reduced and oxidized forms, creating a standard redox potential, involved in a start-up and control mechanisms of initiation and acceleration of alpha, beta minus and beta plus decay of radioactive isotopes of elements any kind of group of bacteria Thiobacillus.
The method leads to the production of polonium, radon, France, radium, actinium, thorium, protactinium, uranium, neptunium, americium and their isotopes as well as nickel, manganese, bromine, hafnium, ytterbium, mercury, gold, platinum, and their isotopes. As radioactive materials containing radioactive chemical elements can be used ore or radioactive nuclear waste cycles…”
http://bt-isotopes.com/
Contact details
actinium.post@gmail.com
tel: +41 22 575 27 33
tel: +41 79 740 25 47 Russian Language
Biochemical method of chemical
elements transmutation and chemical elements isotopes
transformation
ABOUT US
We are a scientific group called “Actinides”. Members of our group have invented a process, which we call MBT –a biochemical method to derive the most valuable elements and theirs isotopes. It’s a revolutionary invention, a breakthrough in the industry of obtaining rare isotopes and elements.
We have brilliant scientists and businessmen in our team, we want to produce rare elements and valuable isotopes is Switzerland. Since it offers the best conditions for business, investments and simple laws for nuclear elements treatment.
RU2563511
MICROBIOLOGICAL METHOD OF TRANSMUTATION OF CHEMICAL ELEMENTS AND CONVERSION OF ISOTOPES OF CHEMICAL ELEMENTS
MICROBIOLOGICAL METHOD OF TRANSMUTATION OF CHEMICAL ELEMENTS AND CONVERSION OF ISOTOPES OF CHEMICAL ELEMENTS
Abstract:
FIELD: biotechnology.
SUBSTANCE: radioactive raw materials containing radioactive chemical elements or their isotopes, are treated with an aqueous suspension of bacteria of Thiobacillus in the presence of elements with variable valence. The radioactive raw materials are used as ores or radioactive wastes of nuclear cycles. The method is implemented to obtain polonium, radon, francium, radium, actinium, thorium, protactinium, uranium, neptunium, americium, nickel, manganese, bromine, hafnium, ytterbium, mercury, gold, platinum, and their isotopes.
EFFECT: invention enables to obtain valuable radioactive elements, to carry out the inactivation of nuclear wastes with the conversion of radioactive isotopes of the waste elements into stable isotopes.
The invention relates to chemical transmutation of radioactive isotopes and transformation, that is to artificially produce some chemical elements from other elements. In particular, the method allows to obtain rare and valuable elements: polonium, radon, francium, radium, and actinides - actinium, thorium, protactinium, uranium, neptunium, and various isotopes of these and other elements.
Known transformation of chemical elements, the formation of new isotopes of elements and new chemical elements during nuclear fission and synthesis of chemical elements used in conventional nuclear rectors, in nuclear power plants (NPPs) in research nuclear reactors, for example, by irradiation of the chemical elements with neutrons or protons, or alpha particles.
A method of obtaining the radionuclide nickel-63 in the reactor from a target comprising obtaining enriched Nickel-62 nickel target, the irradiation target in the reactor, followed by enrichment of irradiated product from nickel-63 at extraction of nickel-64 isotope product (RU 2313149, 2007). An advantage of the method is to obtain a high quality product that is intended for use in self-contained electrical power sources, detectors and other explosives. Reproducibility was confirmed by the analysis of the isotopic composition of elements by mass spectrometry.
However, the method is complicated and unsafe degree requires industrial safety.
It is also known the transmutation of elements - long-lived radioactive nuclides, including those arising in irradiated nuclear fuel (RU 2415486, 2011). The method consists in irradiating neutron flux transmutable material, the irradiation is carried out with neutrons obtained in the nuclear fusion reactions in the pre-formed neutron source plasma, at a certain placement of the scattering medium neutrons. This method is based on the reactions of nuclear fusion in a tokamak is also complex and requires special equipment.
A method of obtaining radionuclides Th-228 and Ra-224, which is also implemented in a reactor technology. The technology is quite complex and has a safety limit (RU 2317607, 2008).
Thus, upon receipt of the chemical elements and their isotopes, in general, are conventionally used nuclear reactions involving nuclear reactors or other sophisticated equipment at high energy costs.
Attempts have been made to solve the problem of obtaining radioactive isotopes in the process of nuclear transmutation of elements more secure manner using the microorganisms. Known in particular isotopes conversion method using microorganisms comprising growing microbial culture Deinococcus radiodurans on a nutrient medium containing the necessary for transmutation of initial isotopic components, and deficient close chemical analogues of the target element. The composition of the medium is introduced, such starting isotopic components which are radioactive and transmutation process can lead to the formation of the target chemical element in the form of a stable or radioactive isotope, which is absorbed by the microbial culture and then remains steady or remains radioactive or decomposed to the desired stable isotope (RU 2002101281 A, 2003). This method does not provide a high yield of the desired isotope, and also requires the use of ionizing radiation as a trigger and response factor supports.
Also known process for the preparation of stable isotopes by nuclear transmutation type of cold fusion elements in microbial cultures (RU 2052223, 1996). The method consists in the fact that the cells of microorganisms are grown in a culture medium deficient isotope target (target isotopes) impact factors contributing to the destruction of the interatomic bonds and leading to an increase in its concentration of free atoms or ions of hydrogen isotopes. The medium is prepared on the basis of heavy water and injected into it scarce for the environment unstable isotopes that decay at the end to form the desired stable isotopes. As a factor that destroys the interatomic bonds using ionizing radiation. This method is based on the use of ionizing radiation, it is not designed for commercial scale requires a high energy and cost.
All of the chemical elements and their isotopes and by-products obtained until now complex and unsafe traditional methods by conventional nuclear reactions in small (sometimes - in micro) amounts clearly insufficient for the energy, industrial, industrial, technical and scientific needs of mankind. Described microbial process for the transmutation of chemical elements allows you to receive all of these chemical elements and their isotopes in almost unlimited quantities, simple to perform, safe for workers and the public, environmentally friendly way that does not require large material flow rates, heat, electricity and heating, while providing this energy, industrial, technical and scientific problems of civilization. These elements and isotopes are enormous reserves of energy, have an extremely high value and selling price on the market.
Microbiological method is proposed transmutation of the chemical elements and isotopes of chemical conversion elements, characterized in that the radioactive feedstock containing radioactive chemical elements or isotopes, treated with an aqueous suspension of bacteria of the genus Thiobacillus, in the presence of any s, p, d, f-elements with variable valency. Selection of elements with variable valence based on the principle of creating a high redox potential. That is, this selection key, or simply on the orientation of these or other elements of variable valency brought into the reaction medium, a redox potential value which is optimal in the range of 400-800 mV (for example, in Examples 1, 2, 3, 4 Eh = 635 mV, 798 mV, 753 mV and 717 mV, respectively).
Items with variable valence, as in the reduced and oxidized forms, creating a standard redox potential, involved in a start-up and control mechanisms of initiation and acceleration of alpha, beta minus and beta plus decay of radioactive isotopes of elements any kind of group of bacteria Thiobacillus.
The method leads to the production of polonium, radon, France, radium, actinium, thorium, protactinium, uranium, neptunium, americium and their isotopes as well as nickel, manganese, bromine, hafnium, ytterbium, mercury, gold, platinum, and their isotopes. As radioactive materials containing radioactive chemical elements can be used ore or radioactive nuclear waste cycles.
According to the inventive method are derived from raw materials containing natural uranium-238 and thorium-232, the following elements:
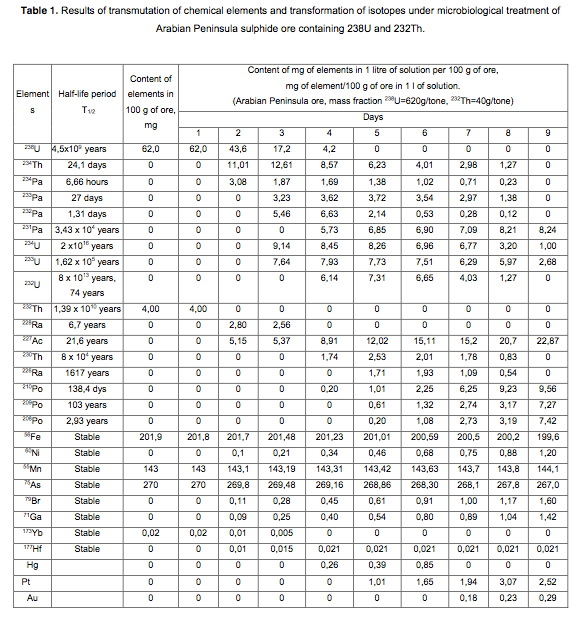
1. Protactinium, actinides, radium isotopes of polonium and various data elements (Tables 1, 2, 3, 4, scheme 1, 2, 3, 4, 5, 6, 7; figures from 1 to 17).
2. Francium (Figures 4, 5, 6, 7, 9, 14).
3. Ytterbium, hafnium, gallium, nickel (Table 1; Figures 2, 3, 4, 5, 6, 7), gold (table 1; figure 6, 7), mercury (Tables 1, 2, scheme 9, 10; Figure 4 , 5, 11), platinum (table 1; scheme 9, 10; figure 4, 5, 6, 7).
4. The iron content in the medium is decreased, there is a nickel (in the original ore nickel was not), the nickel content is increased over time (Table 1) as well as iron assumes alpha particles carried bacterial alpha-radioactive elements, becoming nickel. Separation of the proton nuclei of iron leads to increased manganese content in the medium (in the conversion of iron manganese) and, consequently, to reduce the iron content (Table 1).
5. From polonium, a decomposition product of actinides microbiologically transmutation process elements are obtained various isotopes of thallium, mercury, gold, platinum, including stable (Tables 1, 2, scheme 10, 11; Tables 1 and 2; Figures 1, 2, 3, 4, 5, 6, 7, 11).
6. From obtained rare isotopes of plutonium-239: uranium-235, thorium-231, protactinium-231, Actinium-227 (Scheme 12).
7. Because plutonium 241, which is a by-product of the combustion of uranium in the reactor, obtained rare in nature and industry, and deficient isotopes of americium and neptunium, <241> Am and <237> Np (Scheme 13).
Thus, the described microbial process solves the problem of providing energy and scarce rare materials of various fields of industry, science and technology.
Previously, all of these elements and their different isotopes were produced artificially in small and micro-quantities (grams, milligrams, micrograms or less) in nuclear reactions and processes in a nuclear reactor as decay products of uranium and thorium, and plutonium, radium . Artificially in nuclear reactions isotopes of thorium and uranium have also been obtained. The authors obtained in this manner the following elements: polonium, radon, francium, radium, and actinides - actinium, thorium, protactinium, uranium, neptunium, plutonium, americium, and various isotopes of these elements, as well as various isotopes of thorium and uranium - thorium-227, thorium 228, thorium-230, thorium-234; uranium-231, uranium-232, uranium-233, uranium-234, uranium-235, uranium-236, uranium-239, as well as manganese, nickel, gallium, bromo, hafnium, ytterbium, thallium, mercury, gold, platinum ( cm. schemes 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, and 13 of table 1, 2, 3, 4).
The claimed method of transmutation of chemical elements allows you to receive all of these chemical elements and their isotopes in virtually unlimited quantities.
The described method of transmutation of elements can also inactivate and neutralize nuclear waste, for example, the combustion of nuclear fuel waste (uranium) from the nuclear power plant, containing uranium, plutonium, and their isotopes and fission decay products (isotopic transitions products): isotopes of uranium and plutonium (see diagram. 13), radium and polonium, radioactive isotopes of strontium, iodine, cesium, radon, xenon and other products alpha and beta decay, and spontaneous fission of uranium and plutonium.
It should be noted that certain traditional nuclear reactor methods for making and isolating polonium, radium, actinium, protactinium, neptunium, americium, and their isotopes and of isotopes of thorium and uranium technologically difficult to carry out, are expensive, require complex and expensive equipment and are dangerous to human health and the environment environment, in contrast to the claimed method. Also known conventional nuclear reactor methods for making and isolating polonium, radium, actinium, protactinium, neptunium, americium, and their isotopes of thorium and uranium isotopes do not provide energy needs and other various fields of science and technology in the data of chemical elements and their isotopes.
The claimed method bacteria of the genus Thiobacillus ( e.g., species Thiobacillus aquaesulis or Thiobacillus ferrooxidans ) in the presence of elements with variable valence, initiate and accelerate the natural processes of decay of the radioactive isotope and radioactive transition elements. At the same time, the natural nuclear reactions and isotopic transitions accelerated in thousands, millions and billions of times - depending on the source of the natural half-life of the isotopes of certain chemical elements.
The feedstock used and any raw material containing radioactive elements, namely:
1. Natural uranium and thorium in the form of ores of uranium and / or thorium ores or sand, for example, monazite sand containing thorium phosphates / phosphates; any ore containing impurities thorium, uranium, plutonium in any amounts and ratios to one another.
2. Plutonium (see. The scheme 12, 13), uranium, thorium and other radioactive elements produced in nuclear reactors, including those which are nuclear waste cycles.
3. Any other components and industrial wastes containing a any actinides, mainly, thorium, uranium, plutonium, or as more commonly available and inexpensive in the market, any of these elements in any ratio among themselves.
4. The radioactive decay of plutonium series products, uranium, thorium: radium, radon, polonium.
5. Polonium, which is the product of a microbiological decay actinides transmutation process elements for different rare isotopes of thallium, mercury, gold, platinum, including their stable isotopes.
6. Radioactive products (fragments) of plutonium and uranium fission - radioactive isotopes of strontium, yttrium, cesium, iodine, and other elements; their transmutation is suitable for the purpose of turning them into non-radioactive and non-hazardous to human elements and isotopes for environmental improvements. 7. All these feedstocks (elements) used for the microbial treatment either separately or together in any ratio with each other.
A feed comprising any of the above radioactive elements treated with an aqueous solution of Thiobacillus spp, for example, the type of Thiobacillus aquaesullis or Thiobacillus ferrooxidans, or a mixture thereof in any proportions relative to each other, or any kind of sulfur-oxidizing bacteria in the presence of elements with variable valency, in normal circumstances, the activity of microorganisms.
The method does not require expensive and hazardous to humans and environmental nuclear reactors conducted under conventional conditions in conventional containers at normal ambient temperature (it is quite acceptable values from 4 to 60 degrees Celsius), under normal atmospheric pressure, requires fresh water consumption.
Machinery
In our method, the microorganisms initiate and accelerate the alpha decay (-a), a beta-minus (-β), and beta-plus (+ β) decay (electron capture). Microorganisms captured in the nuclei of heavy elements (mainly in any f-elements and heavy s-elements) protons, alpha particle (two protons and two neutrons) and electrons (beta-minus decay), transferring thus trapped protons, alpha -particles and electrons to other elements, mainly for d- and p-elements, such as arsenic and iron. Also, microorganisms may transfer protons, alpha particles, electrons and positrons to other elements, such as the f-ytterbium element, if present in the medium. Bacterial capture and detachment of protons, alpha particles and electrons occurs at the radioactive elements f-s-group and the group (according to the classification of the periodic system). Also, the bacteria initiate and reproach beta plus (+ β) decay (electron capture) in the nuclei of beta-plus radioactive isotopes of elements of any group, transferring to the core elements of the electron data obtained in the process of beta-minus (-beta) decay of isotopes of other exposed beta-minus decay, or captured at present in the medium of variable valency elements (not radioactive) during their bacterial oxidation.
Bacterial proton transfer (P), alpha particles (α) and electron (e <->) is carried out by elements of d-groups (for example, iron and other) to the elements p-groups (for example, arsenic and others) and s-group elements (strontium, cesium, radium and other).
Bacterial capture and detachment of protons, alpha particles and electrons occurs at alpha- and beta-radioactive isotopes f-group elements, s-p-groups and groups which are themselves naturally (natural) alpha- or beta-radioactive, wherein bacteria initiate and millions and billions of times accelerate the processes of alpha and beta decay.
Bio-alpha decay (-α)
During alpha decay, with loss of two protons nuclei elements f- s-groups and are converted into lighter elements (transition into two cells down the table of the periodic system).
After the capture and separation from the f- and s-components of protons and alpha particles, bacteria carry these protons and alpha particles in the various elements of the d-, p- and s-groups, turning them the other elements - on the following location in the periodic table of chemical elements (transfer to one or two cells on the front table of the periodic system).
In bacterial alpha-particle transfer of f-elements iron, nickel is converted into iron (see. Table 1); in bacterial transferring protons and alpha particles from the f-elements arsenic, arsenic is converted to bromine (see. Table 1); in bacterial transferring protons and alpha particles from the f-elements ytterbium, ytterbium converted into hafnium (see. Table 1).
Bio-beta decay (-β, + β)
Bacteria and provoke many times accelerate both types of beta decay: beta-minus decay and beta-plus decay.
Beta-minus decay (-β) - is a core electron emission, resulting in a proton neutron conversion element to convert the location to the next by a periodic system of chemical elements (shift one cell down the periodic table of elements of the system).
Beta decay plus (+ β) - electron capture nucleus, resulting in the conversion of a proton to a neutron conversion element according to the previous location in the periodic system of chemical elements (transition to single cell backward in the periodic table of the system).
In the process triggered by bacteria and accelerated beta decay, in some cases, there is a consequent emission of so-called delayed neutron - is spontaneous, natural way according to the laws of physics of isotope decay and transitions to give a lighter isotope of a given element. Using the emission mechanism of the delayed neutron allows to further expand the list of received elements and isotopes, and also to predict and regulate the bio-transmutation process (to stop it at the right time).
The bacteria initiate and accelerate beta decay - the emission of an electron and the nucleus of the introduction of the electron to the nucleus (electron capture) of beta-radioactive chemical elements. The bacteria initiate and accelerate the beta decay isotopes of elements as raw materials initially contained in a medium, and isotopes of elements obtained artificially bioprocess bacteria instigated after alpha decay. This fact - beta decay occurring after bacterial-induced alpha decay has great practical significance for the purpose of obtaining scarce energy-important elements and isotopes.
Bacteria gripped and torn away electrons also have more light, as compared with f-elements, nuclei, and just at the beta-minus radioactive isotopes - products ( "fragments") dividing the uranium and plutonium, for example, strontium-90 nuclei, yttrium-90 , iodine-129, iodine-130, cesium-133, cesium-137 and certain other elements which are converted in the process of beta decay into stable elements. In this chemical element in the core a neutron conversion occurs in a proton, and a sequence number offset by one element or two (depending on the initial isotope) cell forward periodic table of elements. This process allows you to radically and environmentally friendly to dispose of highly radioactive waste of nuclear plants and nuclear power plants, ie Nuclear-fuel combustion products that contain radioactive elements - "fragments" of the fission of uranium, plutonium and other transuranic elements - actinides and fission products of thorium, in the case of its use in the thorium nuclear cycle.
An electron captured by bacteria in the beta-minus decay, the bacterium was transferred to a kernel plus beta-radioactive isotopes of elements (in the case of their presence in the environment). In the process go as redox reactions. For example, the latter is converted into iron (II), the latter is converted into arsenic (III) in bacterial electron transfer to arsenic (V) in bacterial electron transfer to the iron (III). Bacterial cell surface charge caused by dissociation of ionic groups of the cell wall, which consists of proteins, phospholipids and lipopolysaccharides. At physiological pH microbial cells, bacteria bear on their surface an excess negative charge which is formed by the dissociation of ionic, preferably acidic groups of the cell surface. The negatively charged surface of the microbial cells from the environment attracts oppositely charged ions, which are under the influence of electrostatic forces tend to approach the ionized groups of the cell membrane.
As a result, the cell is surrounded by an electric double layer (adsorption and diffusion). Charge cell continuously varies depending on the processes occurring in the environment. When exposed to alpha particles, negatively charged cells decreases (in absolute value) and converted into a positive charge, which speeds up the process of beta decay. Next, under the influence of the electrons liberated by beta decay of radioactive elements, as well as the electrons that have fallen out of the elements of variable valence in reduced form in the adsorption layer of microorganisms, the negative charge of microorganisms increases (in absolute value), turns from positive to negative, which accelerates processes of alpha-decay-pulling positively charged protons and alpha particles from the atoms of chemical elements. These processes are accelerating due to electrical interactions negatively and positively charged groups of the surface of the cells with alpha and beta particles, radioactive elements, respectively.
The logarithmic growth stage of microorganisms negative charge of the cell reaches its maximum value, which leads to a maximum rate of transformation, the transformation elements. Processes of conversion of chemical elements can occur both within the bacterial cells and on the cell wall surface of the adsorption layer in the electric double layer.
Thus, the microbial cells, labile changing their charging performance, are accelerating and regulating the system of several kinds of radioactive decay and transformation of one element into another.
To accelerate the process of transmutation of the chemical elements microorganisms when charge microorganisms approaching the isoelectric point in the reaction solution used surfactants (surfactant). Polyampholytes, ionic surfactants, both anionic and cationic surfactant introduced into the reaction medium, by changing the charge cell (charge shift of isoelectric point in the negative or positive direction), promote bacterial initiation and intensification of chemical transmutation (Example 9).
Industrial, scientific and technical importance of the invention
Microbiological method for the transmutation of elements, the acceleration of nuclear reactions and isotopic transitions, allows unlimited quantities of produce valuable and scarce radioactive elements, which are in high demand in the market, technology, industry and research. These elements and isotopes are enormous reserves of energy, have an extremely high value and selling price on the market. The following highlights the small and rare in the nature of the content of chemical elements and their isotopes data, the complexity of their production in nuclear reactors, resulting in their global production is negligible, and the market price is very high. Also described are the application received by the elements and the global demand for them.
Polonium
Polonium is always present in uranium and thorium minerals, but in such minute quantities that getting it from ores known traditional methods impractical and uneconomic. The equilibrium content of polonium in the earth's crust - about 2 x 10 <-14>% by weight. Microquantities polonium extracted from the waste processing of uranium ores. Allocate polonium extraction, ion exchange chromatography and sublimation.
The main industrial method of obtaining polonium is its artificial synthesis by nuclear reactions, which is expensive and unsafe.
Polonium-210 in alloys with beryllium and boron is used for the production of compact and very powerful neutron sources, virtually creating a gamma-radiation (but short-lived because of the small lifetime of the <210> Po: T1 / 2 = 138.376 days) - alpha particles poloniya- 210 give rise to neutrons in nuclei beryllium or boron (α, n) -reaction. This sealed metal ampoules, in which lies covered with polonium-210 ceramic pellets of boron carbide carbide or beryllium. Such neutron sources are light and portable, it is safe to use and very reliable. For example, the Soviet neutron source VNI-2 is a two brass vial diameter and a height of four centimeters, every second radiating up to 90 million neutrons.
Polonium is sometimes used to ionize the gas, in particular air. The first ionization of air is needed to deal with static electricity (at work, when dealing with particularly sensitive equipment). For example, for precision optics made dusting brush.
An important application of polonium is its use in the form of alloys with lead, yttrium or alone for the production of powerful and highly compact source of heat for the stand-alone installations, such as space or polar. One cubic centimeter of polonium-210 releases about 1320 watts of heat. For example, the Soviet space program of self-propelled vehicles "Lunokhod" to heat the instrument compartment heater used polonium.
Polonium-210 can serve as an alloy with the light isotope of lithium (<6> Li) substance that can substantially reduce the critical mass of nuclear charge and serve as a kind of nuclear detonator.
So far, industrial and commercial (market) amounts of polonium were milligrams and grams of polonium.
Radium
At the time of the radium used in compact neutron sources, for this small amount is fused with beryllium. Under the action of alpha radiation from beryllium neutrons are knocked out: <9> Be + <4> He → <12> C + <1> n.
The medicine is used as a source of radium radon, including radon baths for cooking. Radium is used for short-term irradiation in the treatment of malignant diseases of the skin, nasal mucosa, urinary tract.
Low use of radium is due, in particular, with its negligible content in the crust and in the ores, and the high cost and complexity of obtaining artificially in nuclear reactions.
During the time that has elapsed since the discovery of radium - more than a century - around the world managed to get only 1.5 kg of pure radium. One ton of pitchblende, from which the Curies received radium, contained only about 0.0001 gram of radium-226. All natural radium is radiogenic - it arises from the decay of uranium-238, uranium-235 and thorium-232. In equilibrium, the ratio of the uranium-238 and radium-226 in the ore is equal to the ratio of half-periods: (4.468 x 10 <9> s) / (1617) = 2,789 x 10 <6>. Thus, for every three million uranium atoms in nature it represents only one atom of radium. Microbiological method for the transmutation of chemical elements can be obtained from uranium and thorium, radium-226 and other isotopes of radium in virtually unlimited quantities (kilograms, tons) and to extend the scope of radium and its isotopes.
Francium
Currently, Francium and its practical application have salt, due to short half-life. From well-known by far the most long-lived isotope France <223> Fr has a half-life of 22 minutes. However, obtaining microbiologically France transmutation of the chemical elements and fixation devices for the presence of France in treated samples (Figures 4, 5, 6, 7, 9, 14), in the absence of France in the feedstock, the general course of processes proves conversion elements. In the future, it is possible to use in France and other scientific purposes.
Actinium
Actinium is one of the less common naturally radioactive elements. Its total content in the crust of less than 2600 m, while, for example, the amount of radium over 40 Mill. T. In nature, we found 3 actinium isotope <225> Ac, <227> Ac, <228> Ac. Actinium accompanies uranium ores. Getting actinium from uranium ore known traditional methods is impractical because of the paucity of its content in them, and the great similarity with the present there is rare earth elements.
Significant amounts of the isotope <227> Ac get radium irradiation by neutrons in the reactor. <226> Ra (n, γ) → <227> Ra (-β) → <227> Ac. The yield is usually not more than 2.15% of the initial amount of radium. Number actinium in this method of synthesis is calculated in grams. Isotope <228> Ac obtained by irradiation of the isotope <227> Ac neutrons.
<227> Ac beryllium mixed with a source of neutrons.
Ac-Be-sources are characterized by low yield of gamma-ray activation analysis used in the determination of Mn, Si, Al in the ores.
<225> Ac is used to obtain <213> Bi, as well as for use in radioimmunotherapy.
<227> Ac can be used in radioisotope power sources.
<228> Ac is used as a tracer in chemical research due to its high-.beta.-radiation.
A mixture of isotopes <228> Ac- <228> Ra is used in medicine as an intense source of gamma-rays.
Actinium can be a powerful source of energy that has not yet been applied because of the high cost of actinium and small amounts of actinium obtained by known methods, and because of the complexity of its receipt by known methods. All the traditional techniques for making and isolating actinium are expensive, uneconomical and dangerous to human health and the environment. Getting actinium microbiological method of transmutation of chemical elements produces Actinium isotopes and cheap at cost and safe way in unlimited quantities (kilograms, tons, tons, etc.).
Protactinium
Due to the small content in the earth's crust (the content of the Earth's mass is 0.1 billionth of a percent) of the element to date it has a very narrow application - the addition to nuclear fuel. From natural sources - residues from the processing of pitchblende - conventional methods can only protactinium-231 (<231> Pa). In addition, the <231> Pa in the traditional way can be obtained by irradiation of thorium-230 (<230> Th) slow neutrons:
Isotope <233> Pa is also derived from thorium:
As an additive to the nuclear fuel material is added at the rate of protactinium protactinium 0.34 grams per 1 ton of uranium, which is very significantly increases the energy value of uranium and uranium combustion efficiency (a mixture of uranium and protactinium). Get the protactinium microbiological method of transmutation of chemical elements produces protactinium cheap at cost and safe way in unlimited quantities (kilograms, tons, tons, etc.). Get the protactinium microbiological method of transmutation of chemical elements decide on the availability of cheap energy, raw materials and energy products with high efficiency, and meets the needs of protactinium in other areas of science and technology.
Thorium
Different isotopes of thorium (thorium-227, thorium-228, thorium-230, thorium-234 and others), having different half-lives that are not contained in natural thorium obtained microbiological method of transmutation of chemical elements, are of interest for research purposes, and It is of interest as well as sources of energy and raw material for other elements and isotopes.
Uranium isotopes
Currently, 23 known artificial radioactive uranium isotopes with mass numbers from 217 to 242. The most important and valuable isotopes of uranium - uranium-233 and uranium-235. U-233 (<233> U, T1 / 2 = 1.59 x 10 <5> s) obtained by the irradiation by neutrons of thorium-232 and is able to divide exposed to thermal neutrons, making it a promising fuel for nuclear reactors, but this process is very complicated, expensive and environmentally hazardous. The content of valuable isotope uranium-235 (<235> U) in natural uranium is small (0.72% of natural uranium), and its traditional separation from other uranium isotopes (eg, laser centrifugation) and the selection is associated with great technical, economic and environmental difficulties as costly, expensive and complex equipment, and safe for humans and the environment. The isotope uranium-233 (<233> U) in natural uranium is not contained, and its traditional reception in nuclear reactors is associated with the same difficulties and dangers.
Uranium is widely distributed in nature. The uranium content in the crust is 0.0003% (wt.) concentration in the sea water 3 g / l. The amount of uranium in the layer thickness of the lithosphere 20 km is estimated to be 1.3 x 10 <14> m. The world uranium production in 2009 was 50,772 tons, the world's resources for 2009 amounted to 2,438,100 tons. Thus, the world's uranium reserves and the world production of natural uranium are large enough. The problem is that most of the reserves and production (99.27%) are in the natural isotope uranium-238, uranium (respectively, the percentage of isotopes in natural uranium), ie the least useful and least energetic isotope of uranium. Besides the traditional separation of uranium isotopes from each other (in this case, the uranium-235 from uranium-238) is extremely difficult, expensive and environmentally unsafe. According to OECD data, the world's 440 operating nuclear reactors for commercial use, which consumes annually 67 ths. Tons of uranium. This means that its production provides only 60% of its consumption (the rest is extracted from old nuclear warheads).
The most valuable in this case, the isotopes of uranium - uranium-233 and uranium-235 (the nuclear fuel) for which and reused after reprocessing of spent fuel rods from nuclear power plants and de-alerting of nuclear warheads. Cores <238> U are divided only in the capture of fast neutrons with an energy of at least 1 MeV. Cores <235> U and <233> U divided the capture as slow (thermal) and fast neutrons, and are divided spontaneously, which is particularly important and valuable.
Microbiological method for the transmutation of chemical elements allows virtually unlimited quantities produced from natural uranium (from the isotope uranium-238), a rare and valuable isotopes of uranium - uranium-232, uranium-233, uranium-234, uranium-235, uranium-236, as well as other valuable chemical elements and their isotopes: neptunium-236, neptunium-237, neptunium-238, plutonium-236, plutonium-238, americium-241, protactinium-231, protactinium-234, thorium-227, thorium-228, thorium-230 actinium-227, radium-226, radium-228, radon-222, polonium-209, polonium-210. Industrial, technical and energy value as well as the selling market value of these elements obtained much higher than the original element - uranium-238.
Neptunium
Neptunium is encountered on Earth only in trace amounts, it has been artificially produced from uranium by nuclear reactions.
The content of <237> of Np in the irradiated uranium fuel is low, and is estimated to be approximately equal to 0.1-0.3% of the resultant plutonium or 10 <-4> x 10 <-6>% by weight of the uranium content. When using uranium fuel enriched in the isotopes <235> U and <236> U, Np-237 is produced mainly by the following nuclear reaction:
By neutron irradiation of neptunium-237 is obtained by weight amounts of isotopically pure plutonium-238, which is used in small radioisotope power sources, RTGs (RTG - Radio-isotope thermoelectric generator), in pacemakers, as a heat source in radioisotope power sources and neutron sources .
The critical mass of neptunium-237 is about 57 kg of a pure metal, and thus the isotope can be practically used to produce nuclear weapons.
Americium
Americium-241 is produced by neutron irradiation of plutonium:
Americium-241 - valuable rare chemical elements and isotopes, its traditional reception in nuclear reactors is associated with the usual for actinide complex and expensive as a result of americium has a larger market value, demand and can be used in various fields of science, industry and technology.
Microbiological method for the transmutation of chemical elements allows to get practically unlimited quantities of neptunium-236, neptunium-237, neptunium-238, plutonium-236, plutonium-238, americium-241 and other isotopes of neptunium, plutonium and americium.
Conventional short designations in the following diagrams and tables:
Uranium-238, <238> U - here - 238 - is the relative atomic mass, ie, the total number of protons and neutrons.
P - proton.
Or N n - a neutron.
alpha - alpha particle, ie, two protons and two neutrons.
(-Α) - alpha particle emitted from the atom (from the element) in our reactions, with the sequence number (nuclear charge) is decreased by two units, and the element is converted into lighter disposed through a cell in the periodic table of elements of the periodic (shift of two cells back). Relative atomic mass is then decreased by four units.
Beta decay - making, in which the element (nuclear charge) serial number of changes to the unit and the relative atomic mass (the total number of protons and neutrons) remains constant.
(+ Β) - emission of a positively charged positron particles, or the seizure of a negatively charged electron is the core: in both cases, the serial number (nuclear charge) of the element is reduced by one.
Observed phenomenon of emission of so-called "delayed neutrons" (usually one or two) after beta decay. At the same time, the new formed by the beta decay of a chemical element, after the emission of delayed neutrons (neutrons) retains its new location, and the cell in the periodic table of the system elements, as it saves the nuclear charge (number of protons), but lost in the atomic weight, forming new , lighter isotopes.
(-n) - «Delayed neutron" neutron emitted from an atom after beta decay, and the atomic weight of the new element is reduced by one.
(-2n) - Two "delayed neutrons", emitted from an atom after beta decay, the atomic mass of the new element is reduced by two units.
(Ă) - «retarded» alpha particle (kind of isotope decay) emitted from the atom (element) after beta decay. At the same sequence number (nuclear charge) decreases by two units, and the relative atomic mass of the element is reduced by 4 units.
There is another transmutation of the chemical element (shift of two cells on the back table of chemical elements of the periodic system).
T1 / 2 and T - the half-life of the isotope element.
The authors conducted a series of successful reproducible experiments with a variety of ores and raw materials. Raw materials containing radioactive elements, treated with an aqueous solution of Thiobacillus genus of bacteria in the presence of elements with variable valence any s, p, d and f elements, creating a standard redox potential (eg, Sr <2+>, nitrogen N <5 +> / N <3->, sulfur S <6 +> / S <2-> arsenic As <5 +> / As <3+>, iron Fe <3 +> / Fe <2+>, manganese Mn <4+> / Mn <2+>, molybdenum Mo <6 +> / Mo <2+>, cobalt Co <3 +> / Co <2+>, vanadium V <5 +> / V <4+>, etc.). various bacteria of the genus
Thiobacillus have been used, and iron-sulfur-oxidizing bacteria (thermophilic and others) involved in redox processes of metals, always achieved positive effect. The authors conducted experiments in 2536.
The experimental data is statistically processed (see. Table 1, 2, 3, 4) and are reflected in the schemes produce microbiological method of uranium-238 (238U) and thorium-232 variety of isotopes of uranium, protactinium, thorium, actinium, radium, polonium and other elements (see FIG. 1 to 17, schemes 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13).
Schemes reactions and isotopic transitions do not contradict and confirm the existing theory of radioactive decay.
Example 1.
For transmutation of the chemical elements and receiving elements and isotopes new feedstock microbial treatment using Saudi sulfide ores containing uranium and thorium (Table 1, Figures 1, 2, 3, 4, 5, 6, 7). Saudi ore also contained the elements phosphorus, arsenic, vanadium, mostly in oxidized form (phosphates, arsenates, vanadates), and iron - both in an oxidized and in a reduced form. Therefore, to create a high redox potential in the fermentation raw material treated with microorganisms Thiobacillus acidophilus strain DSM-700 in aqueous solution with a variable valence elements present in the solution in a reduced form: Mn <+4>, Co <+2>, Fe <+2 >, N <-3>, S <-2> (in salt form), in their total weight 0.01% by weight of the medium.
When growing microorganisms Thiobacillus acidophilus strain 700 DSM-used standard culture media (e.g., Waksman and Leathen environment for Thiobacillus ferrooxidans, and 9K medium medium other iron and sulfur-oxidizing bacteria). The standard nutrient medium were added variable valency elements - transelementy (electrons carrying elements, e.g., Mg, Mn, Co, Mo, Zn, Cu, Fe in the form of salts) in their total weight 0.01% by weight of the medium, organic materials hydrolysis products For example, hydrolysis of fish waste, meat, or the timber (2% by weight of the medium) and raw material (uranium or thorium or ore containing radioactive waste in an amount of 1.5% by weight of the medium). The fermentation medium comprising 10% of raw materials (ores) was added 10% solution of the culture medium with optional autotrophic microorganisms selected in the exponential growth phase.
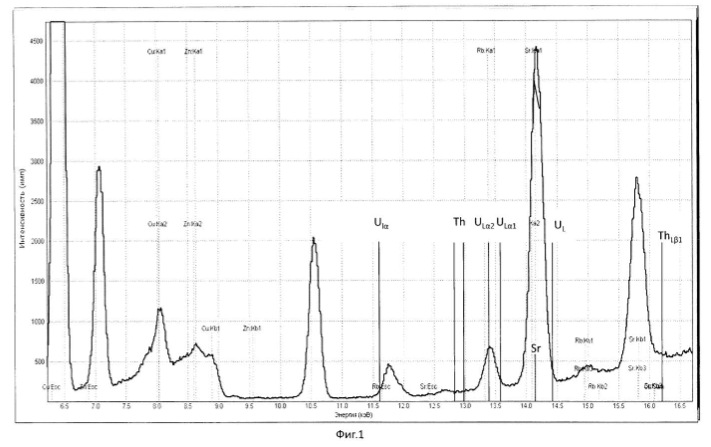
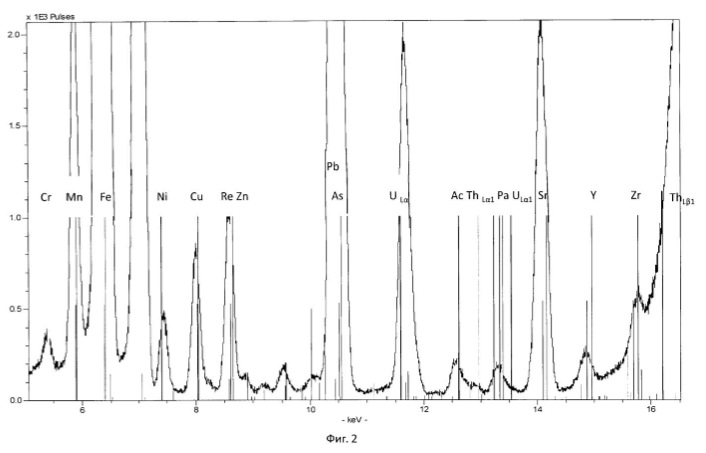
Transmutation process was carried out in ten fermentation shake flasks. PH of the solution was adjusted to 10 normal sulfuric acid, pH of the solution was maintained in the range 0.8-1.0 in. The temperature of the process of 28-32 degrees Celsius. The redox potential (Eh) of the solution in the logarithmic transmutation process step is 635 mV. stirring rate 300 rpm. The ratio of solids to liquid was 1:10 (100 grams ore per liter aqueous solution). Every day, every 24 hours was measured by pH and Eh of the solution, the concentration of chemical elements and isotopes in the solution, and track the activity of microorganisms. The process was conducted for nine days. Use the method of analysis of aqueous solutions and ore: for the determination of elements using X-ray fluorescence method, the type of devices: of CYP-02 "Wren PV"; S2 PICOFOX. Also we are using atomic-absorption method. The isotopic composition was determined by mass spectroscopy.
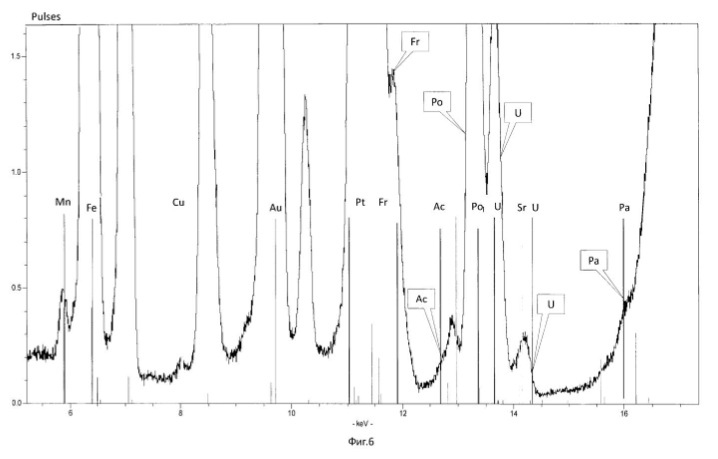
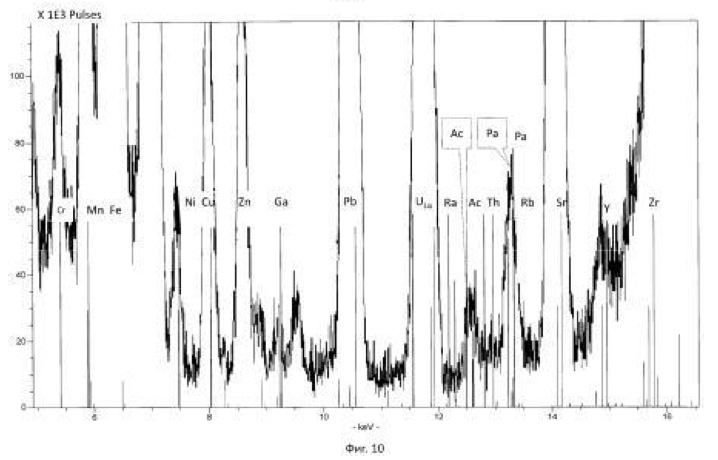
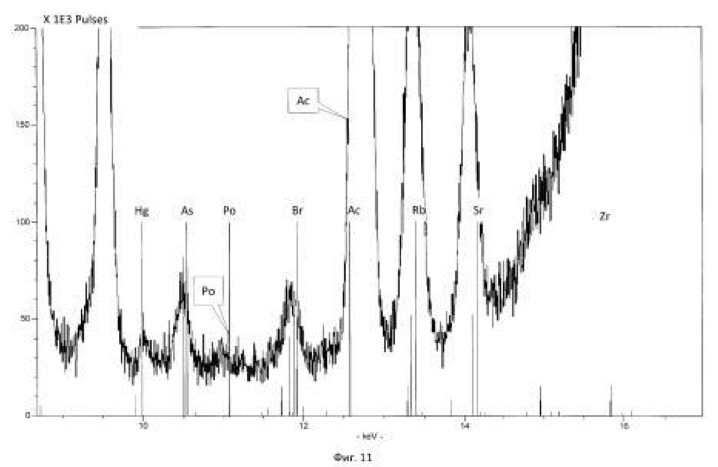
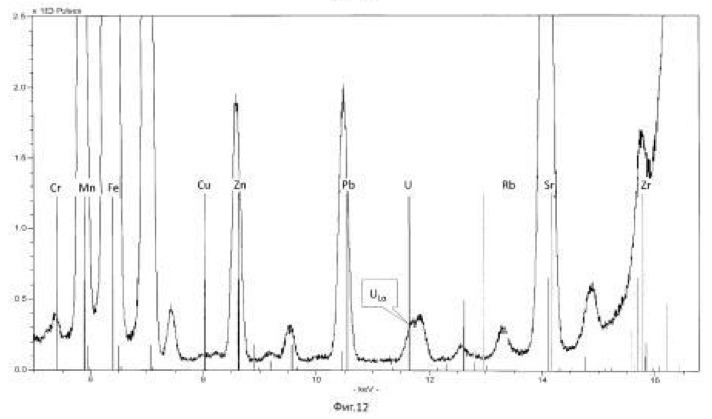
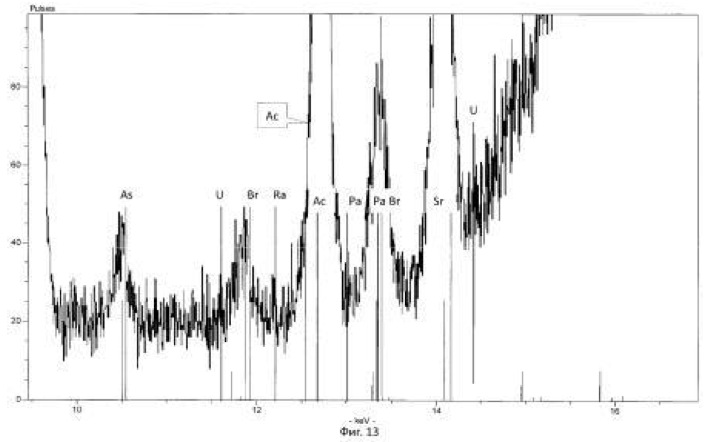
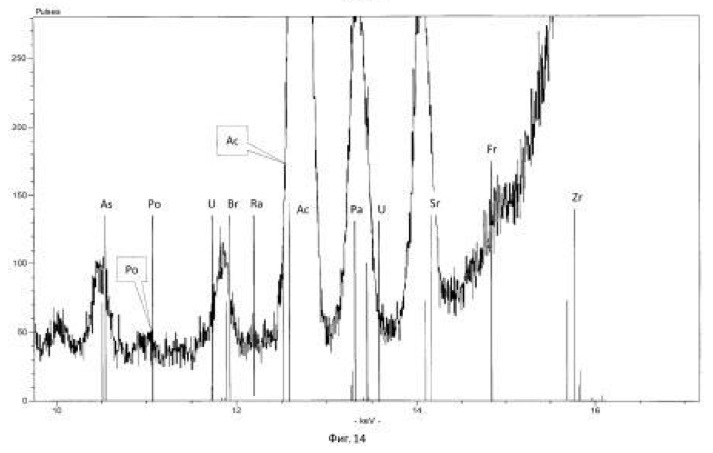
Charging characteristics of microbial cells was determined by electrophoretic mobility in an automatic microscope Parmoquant-2. According to the devices determined by the qualitative and quantitative composition of the end products. The results of these experiments and statistically processed according to the process of time are shown in Table 1. FIG. 1 shows a spectrogram of the original ore Saudi Arabia without microbiological treatment and without transformation of chemical elements. Figures 2, 3, 4, 5, 6, 7 show spectrogram analysis transmutation of the chemical elements in the microbiological treatment of ore Saudi Arabia, depending on the time of the process in 48 hours (2 hours), 72 hours (3 days), 120 hours (5 days), 120 hours (5 days) after 168 hours (7 days) after 192 hours (8 days), respectively.
Scheme 1. Getting the microbiological method of uranium-238 (<238> U) of different isotopes of uranium, protactinium, thorium, actinium, radium, polonium:
Figure 2. Get the protactinium-231 (<231> Pa) microbiological method of uranium-238 (<238> U) in various ways.
Scheme 4. Obtaining thorium-230 (<230> Th) microbiological method of uranium-238 (<238> U).
Next, the process stops or (and secreted <230> Th), if the thorium-230 is the ultimate goal of the process. Or, the process continues to produce valuable and rare radioactive isotopes of radium (<226> Ra), radon, astatine, polonium, bismuth, lead:
Figure 5. Production of Actinium-227 (<227> Ac) microbiological method of uranium-238 (<238> U) in various ways.
Figure 6. Getting radium-226 (<226> Ra) and radium-228 (<228> Ra) microbiological method of uranium-238 (<238> U) (see. 6-1) and from natural thorium-232 (<232> Th ) (2.6 cm), respectively.:
Scheme 7. Getting the most valuable and stable isotopes of polonium (<210> Po, <209> Po, <208> Po) microbiological method of uranium-238 (<238> U).
Figure 8. The preparation of various isotopes of thorium, actinium, radium, polonium microbially produced from natural thorium-232 (<232> Th):







Example 2.
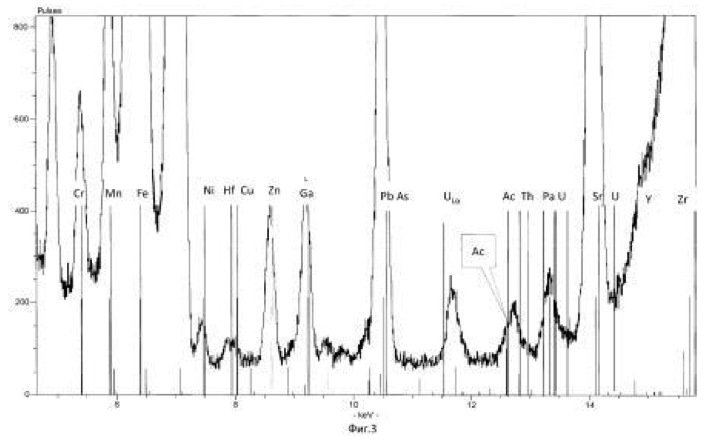
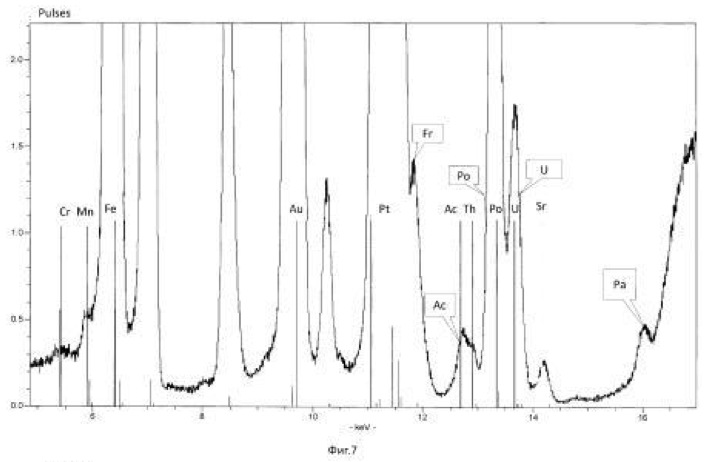
The method of the process is the same as in Example 1. For transmutation of the chemical elements and receiving elements and isotopes new feedstock microbial treatment uranium ore used North-West Africa containing uranium, thorium, arsenic and sulfur in reduced form (metal sulphides, arsenides, sulphoarsenides). Therefore, to create a high redox potential feedstock treated microorganisms Thiobacillus aquaesulis strain DSM-4255 in an aqueous solution with a variable valence elements, in solution in the oxidized form: N <+5>, P <+5> (in the form of phosphates), As <+5>, S <+6>, Fe <+3>, Mn <+7>, their total weight 0.01% by weight of the medium. The redox potential (Eh) of the solution in the logarithmic transmutation process step is 798 mV. The temperature of the process of 30-35 degrees Celsius, pH 2-2.5 environment. The time of the twenty-day process. The results of these experiments and statistically processed according to the process of time are shown in Table 2.
Spectrograms analysis transmutation of chemical elements in microbial processing of uranium ore of the North-West Africa, depending on the time of the process, within 24 hours (1 day), after 144 hours (6 days), after 168 hours (7 days), through 192 hours (8 days) after 480 hours (20 days) are shown in figures 8, 9, 10, 11, respectively.
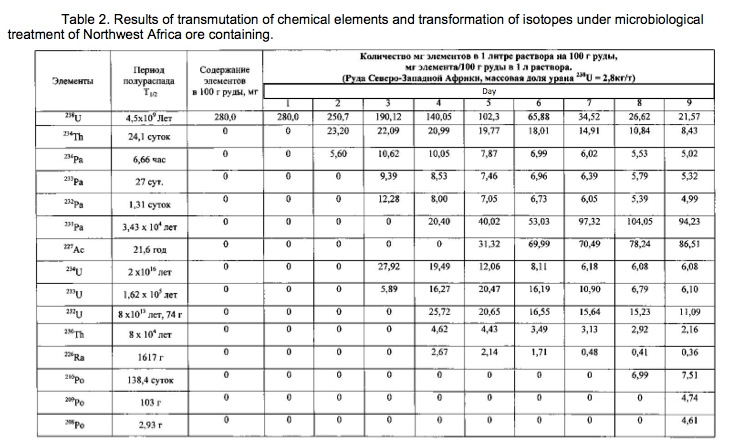
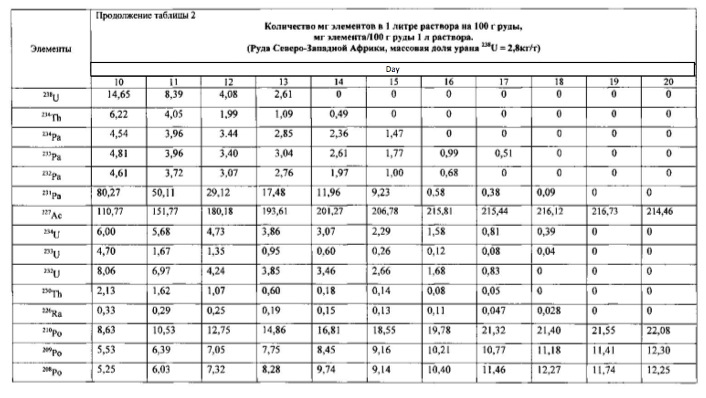








Figure 2. Production of uranium-233 (<233> U) microbiological method of uranium-238 (<238> U) in various ways.
Scheme 3. Get the protactinium-231 (<231> Pa) microbiological method of uranium-238 (<238> U) in various ways.
Scheme 4. Obtaining thorium-230 (<230> Th) microbiological method of uranium-238 (<238> U).
Next, the process stops or (and secreted <230> Th), if the thorium-230 is the ultimate goal of the process. Or, the process continues to produce valuable and rare radioactive isotopes of radium (<226> Ra), radon, astatine, polonium, bismuth, lead:
Figure 5. Production of Actinium-227 (<227> Ac) microbiological method of uranium-238 (<238> U) in various ways.
Diagram 6-1. Getting radium-226 (<226> Ra) microbiological method of uranium-238:
Scheme 7. Getting the most valuable and stable isotopes of polonium (<210> Po, <209> Po, <208> Po) microbiological method of uranium-238 (<238> U). Further transformation path elements and isotopes to the <210> Po, <209> Po, <208> Po identical 7-1 scheme.
Example 3.
The method of the process is the same as in
Example 1. For chemical transmutation and producing new
elements and isotopes as raw material for the microbial
treatment used uranium ore Jordan containing elements uranium,
thorium, phosphorus, arsenic, iron, vanadium in an oxidized
form (phosphates, arsenates, vanadates) and in reduced
potential of the raw materials processed by microorganisms
Thiobacillus halophilus strain DSM-6132 in aqueous solution
with a variable valence elements having redox ability: Rb
<+1>, Sr <+2>, S <0> / S < -2>, Re
<+4> / Re <+7>, As <+3> / As <+5>, Mn
<+4> / Mn <+7>, Fe <+2> / Fe <+3 >, N
<-3> / N <+5>, P <+5>, S <-2> / S
<+6> their total weight 0.01% by weight of the medium.
The redox potential (Eh) of the solution in the logarithmic
transmutation process step is 753 mV.
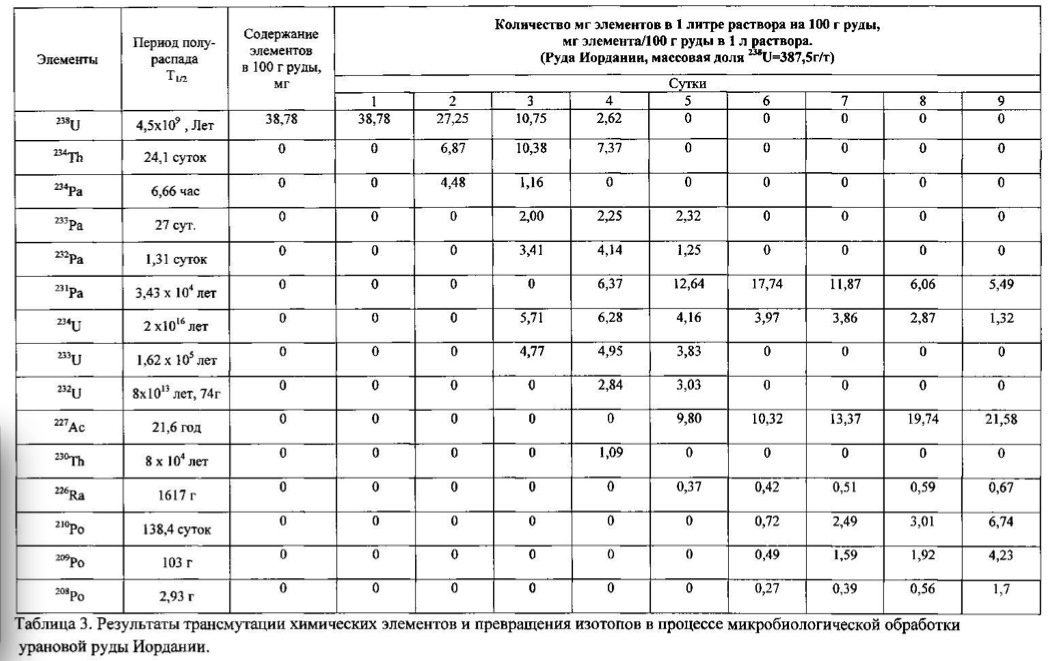
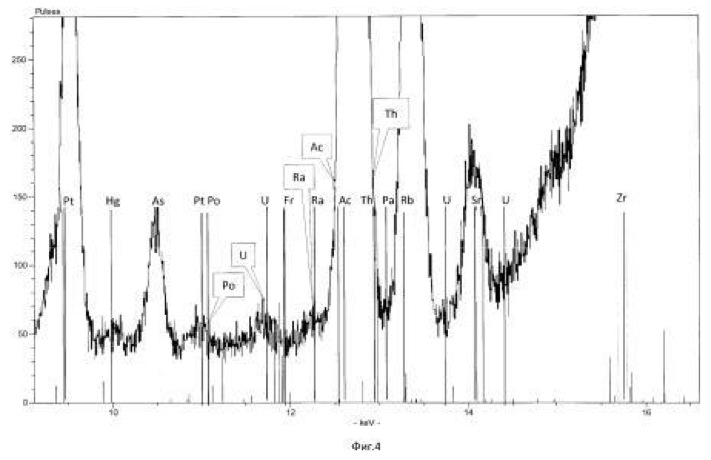
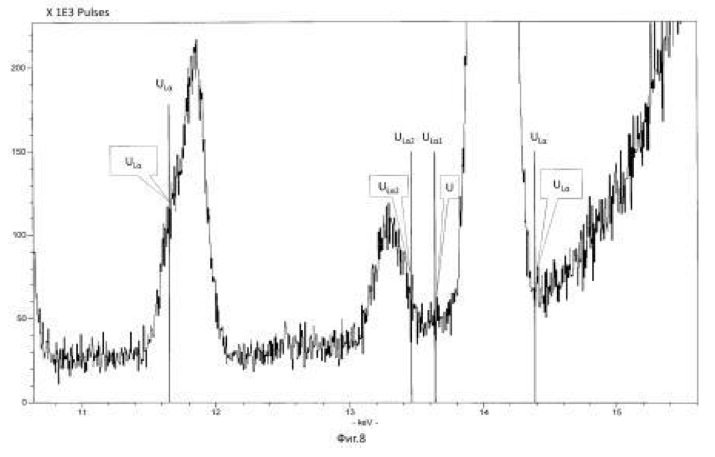
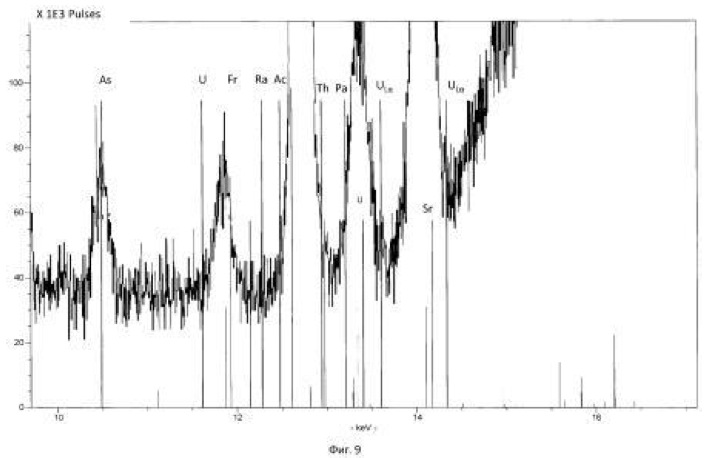
The temperature of the process of 28-32 degrees Celsius, pH 2.0-2.5 environment. The time of the twenty-day process. The results of these experiments and statistically processed according to the process of time are shown in Table 3. Spectrogram analysis transmutation of the chemical elements in the microbiological treatment Jordan uranium ore depending of process time, through 192 hours (8 days), are shown in Figures 12, 13, 14, 24 hours (1 day), 120 hours (five days) respectively.
The temperature of the process of 28-32 degrees Celsius, pH 2.0-2.5 environment. The time of the twenty-day process. The results of these experiments and statistically processed according to the process of time are shown in Table 3. Spectrogram analysis transmutation of the chemical elements in the microbiological treatment Jordan uranium ore depending of process time, through 192 hours (8 days), are shown in Figures 12, 13, 14, 24 hours (1 day), 120 hours (five days) respectively.
 form.
Therefore, to create a high redox
form.
Therefore, to create a high redox
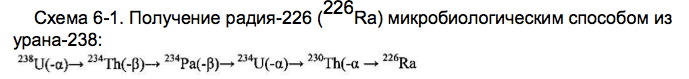

Scheme 3. Get the protactinium-231 (<231> Pa) microbiological method of uranium-238 (<238> U) in various ways.
Scheme 4. Obtaining thorium-230 (<230> Th) microbiological method of uranium-238 (<238> U).
Next, the process stops or (and secreted <230> Th), if the thorium-230 is the ultimate goal of the process. Or, the process continues to produce valuable and rare radioactive isotopes of radium (<226> Ra), radon, astatine, polonium, bismuth, lead:
Figure 5. Production of Actinium-227 (<227> Ac) microbiological method of uranium-238 (<238> U) in various ways.
Diagram 6-1. Getting radium-226 (<226> Ra) microbiological method of uranium-238:
Scheme 7. Getting the most valuable and stable isotopes of polonium (<210> Po, <209> Po, <208> Po) microbiological method of uranium-238 (<238> U).
Example 4.
The method of the process is the same as in Example 1. For chemical transmutation and producing new elements and isotopes in the feedstock used for the microbial treatment monazite sand containing thorium Indian ocean coast, comprising elements thorium, phosphorus, arsenic, silicon, aluminum, cerium and other lanthanides, and mainly in the reduced form. Therefore, to create a high redox potential feedstock treated microorganisms Thiobacillus ferrooxidans strain DSM-14882 in an aqueous solution with a variable valence elements, in solution in the oxidized form: N <+5>, P <+5>, As <+5>, S <+6>, Fe <+3>, Mn <+7>, their total weight 0.01% by weight of the medium. The redox potential (Eh) of the solution in the logarithmic transmutation process step is 717 mV. The temperature of the process of 28-32 degrees Celsius, pH 1.0-1.5 environment. The timing of the process for ten days. The results of these experiments and statistically processed according to the process of time are shown in Table 4.
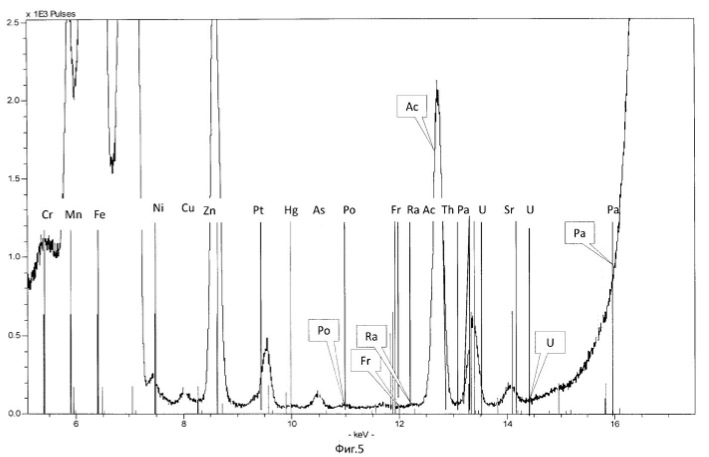
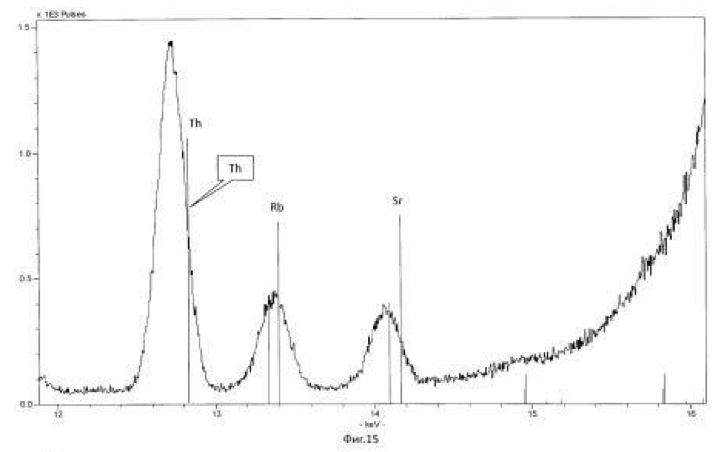
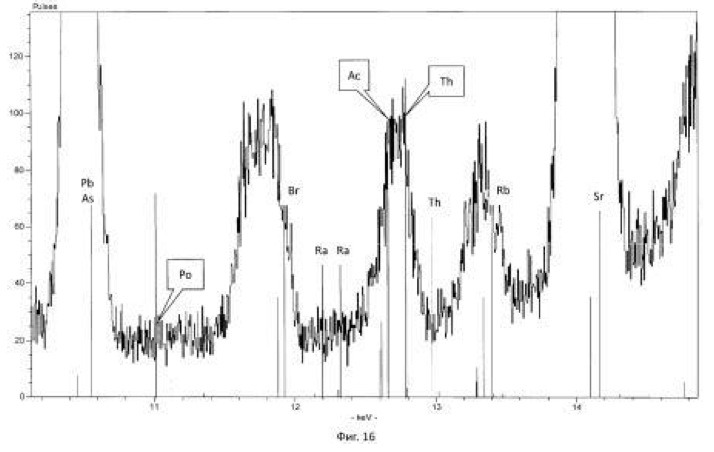
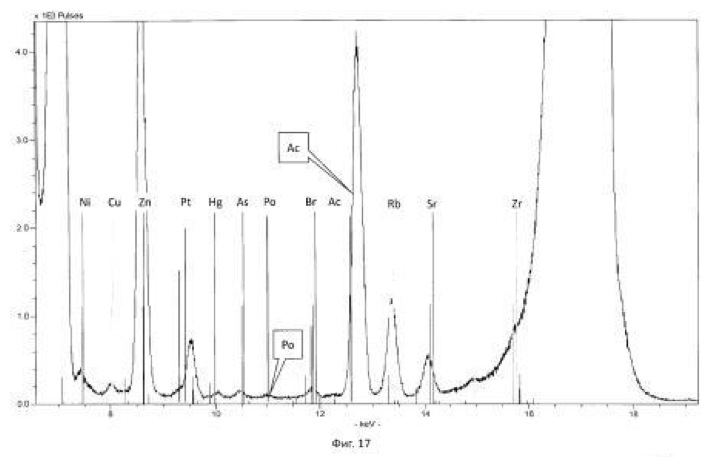
Spectrogram analysis transmutation of the chemical elements in the microbiological treatment of the thorium-containing sand Indian ocean coast, depending on the time of the process, after 24 hours (1 day), 120 hours (five days) after 240 hours (ten days) are shown in Figures 15, 16 17, respectively.
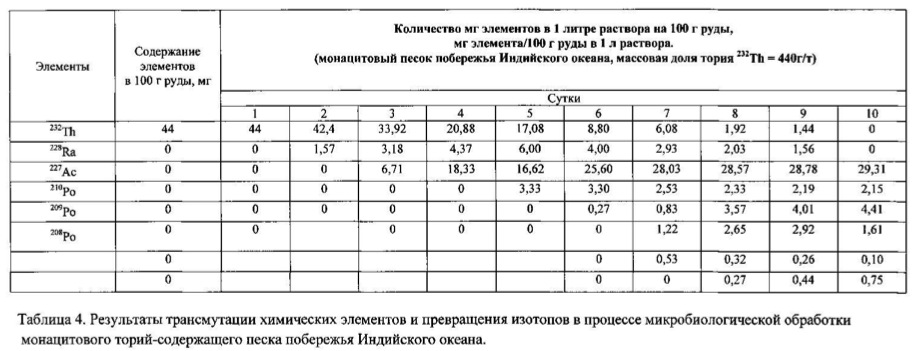



Diagram 6-2. Preparation of radium-228 (<228> Ra) microbially produced from natural thorium-232:
Figure 8. The preparation of various isotopes of thorium, actinium, radium, polonium microbially produced from natural thorium-232 (<232> Th):
Example 5.
The method of the process is the same as in Example 1. For chemical transmutation and producing new elements and isotopes in the feedstock used for the microbial treatment of polonium-209, obtained in our process of actinides turning (decaying) further mercury isotopes, gold, and platinum (Scheme 10). Raw materials processed by microorganisms Thiobacillus aquaesulis strain DSM-4255 in aqueous solution with a variable valence elements having redox ability: Rb <+1>, Sr <+2>, S <0> / S <-2>, Re <+4 > / Re <+7>, As <+3> / As <+5>, Mn <+4> / Mn <+7>, Fe <+2> / Fe <+3>, N <-3> / N <+5>, P <+5>, S <-2> / S <+6> their total weight 0.01% by weight of the medium. The redox potential (Eh) of the solution in the logarithmic transmutation process step is 698 mV. The temperature of the process of 28-32 degrees Celsius, pH 2.0-2.5 environment. The time of the twenty-day process.
Based on the experimental and statistical data processed by the authors derived the following scheme:
Example 6.
The method of the process is the same as in Example 1. For chemical transmutation and producing new elements and isotopes in the feedstock used for the microbial treatment of polonium-208, obtained in our process of actinides turning (decaying) further mercury isotopes, gold, and platinum (Scheme 11). Raw treated microorganisms Thiobacillus ferrooxidans strain DSM-14882 in an aqueous solution with a variable valence elements having redox ability: Rb <+1>, Sr <+2>, S <0> / S <-2>, Re <+4 > / Re <+7>, As <+3> / As <+5>, Mn <+4> / Mn <+7>, Fe <+2> / Fe <+3>, N <-3> / N <+5>, P <+5>, S <-2> / S <+6> their total weight 0.01% by weight of the medium. The transmutation process solution in the logarithmic stage Eh = 753 mV. The microorganisms employed temperature of the process of 28-32 degrees Celsius, pH 1.0-1.5 environment. The time of the twenty-day process. Based on the experimental and statistical data processed by the authors derived the following scheme:
Scheme 11. Preparation of stable isotopes of mercury, thallium, platinum (<195> Pt) and gold (<197> Au) microbiological method to initiate and accelerate the reaction of the polonium-208:
Example 7.
The method of the process is the same as in Example 1. For transmutation of the chemical elements and receiving elements and isotopes new feedstock microbial treatment plutonium samples used to convert the plutonium-239 in U-235, protactinium-231, and actinium-227 (Scheme 12). Raw materials processed by microorganisms Thiobacillus thioparus strain DSM-505 in an aqueous solution of elements with variable valence having redox ability: Rb <+1>, Sr <+2>, S <0> / S <-2>, Re <+4 > / Re <+7>, As <+3> / As <+5>, Mn <+4> / Mn <+7>, Fe <+2> / Fe <+3>, N <-3> / N <+5>, P <+5>, S <-2> / S <+6> their total weight 0.01% by weight of the medium. The redox potential (Eh) of the solution in the process of transmutation logarithmic stage of the process of transmutation Eh = 759 mV. The temperature of the process of 28-32 degrees Celsius, pH 2.0-2.5 environment. The time of the twenty-day process. Based on the experimental and statistical data processed by the authors derived the following scheme:
Scheme 12. Production of uranium-235, thorium-231, protactinium-231 and actinium-227 microbiological method with the acceleration of plutonium-239 decay reactions (may use weapons-grade plutonium, or plutonium - a byproduct of the combustion of nuclear fuel rods NPP to be waste):
You can stop the process at any stage, to form <235> U, or <231> Th, or <231> Pa, or <227> Ac, or mixtures thereof in various proportions. Or, you can continue the process of transformation of elements and isotopes of actinium-227 to <210> Po, <209> Po, <208> Po, to give the intermediate elements, according to the scheme 7-1.
Example 8.
The method of the process is the same as in Example 1. For transmutation of the chemical elements and receiving elements and isotopes new feedstock microbial treatment plutonium samples used to convert plutonium-241, americium-241 and Np-237 (Scheme 13). <241> Pu - a byproduct of nuclear reactions at nuclear power plants burning fuel elements, subject to utilization, taken as a nuclear waste and industrial by-product of uranium combustion. Raw materials processed by microorganisms Thiobacillus tepidarius strain DSM-3134 in aqueous solution with a variable valence elements having redox ability: Rb <+1>, Sr <+2>, S <0> / S <-2>, Re <+4 > / Re <+7>, As <+3> / As <+5>, Mn <+4> / Mn <+7>, Fe <+2> / Fe <+3>, N <-3> / N <+5>, P <+5>, S <-2> / S <+6> their total weight 0.01% by weight of the medium. Eh = 736 mV. The temperature of the process of 28-32 degrees Celsius, pH 2.0-2.5 environment.
Scheme 13. Getting americium-241 (<241> Am) and neptunium-237 (<237> Np) microbiological method of plutonium-241, with the initiation and acceleration of decomposition reactions:
The process can be stopped or slowed down at the stage of americium-241 with the selection of the latter.
Example 9.
This example shows the intensification of the process of transmutation of chemical elements in its deceleration when the limiting factors. The method of the process and the same raw materials as in Example 2. Control option: The raw material was also used uranium ore North-West Africa, but unlike Example 2 consisted in high content of ore in the solution: solid phase ratio (ore) to the liquid phase is 1: 3 (100 grams of ore per 300 ml of aqueous solution ). Raw treated microorganisms Thiobacillus aquaesulis strain DSM-4255 in an aqueous solution with a variable valence elements, in solution in the oxidized form: N <+5>, P <+5> (in the form of phosphate), As <+5>, S <+ 6>, Fe <+3>, Mn <+7>, their total weight 0.01% by weight of the medium, as in example 2. Eh = 410 mV. The temperature of the process to 30-35 degrees Celsius, pH 2.0-2.5 of the medium. The time of the twenty-day process. The charge of bacteria close to zero. The electrophoretic mobility (EPM) of microbial cells is equal to 0,01 V <-1> × cm <2> × s <-1>.
The initial uranium-238 content in the medium was 280 g / l. On the fifth day of the process the uranium-238 content dropped to 200.52 mg / L, but protactinium-231, actinium-227 and polonium isotopes were detected in the medium, wherein the detected isotopes thorium-234, protactinium, 234, 233, protactinium, uranium -234 (primary products of transmutation of uranium-238). Transmutation process, and uranium-238 cells and formation of new isotopes were retarded in time in comparison with Example 2, in which the solid phase ratio (ore) to the liquid phase was 1:10 (100 grams of ore per 1000 ml aqueous solution). Slowing the process due to the high concentration of metal ions in the solution with a small amount of water to the ore. Experimental variant: The same solution is limited by the water in which the solid phase ratio (ore) to the liquid phase is 1: 3 (100 grams of ore per 300 ml of aqueous solution) was further introduced 0,001 g / l polyampholyte - polyacrylic acid, caprolactam ( the ratio of acrylic acid to caprolactam 9: 1). The electrophoretic mobility (EPM) of microbial cells is equal to 0,89 V <-1> × cm <2> × s <-1>, microorganisms charge moved from the isoelectric point in the negative direction.
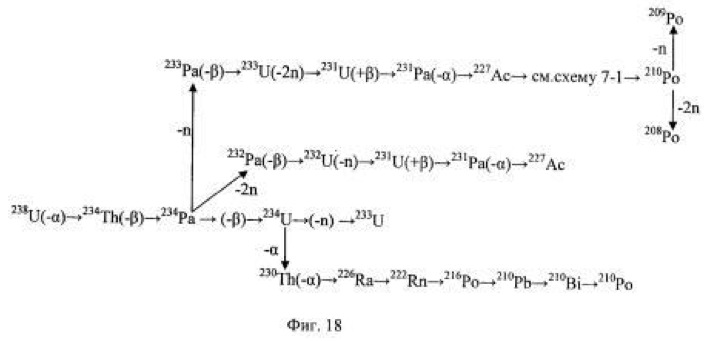
Eh = 792 mV On the fifth day content in uranium-238 solution was equal to 149.40 mg / L, there were isotopes - products of further decay: uranium-232, uranium-233, protactinium-231, Actinium-227, radium-226, polonium -210, 209 and 208 - all in large quantities. There has been a process of acceleration. On the basis of experimental data, a general overview of the various areas and uranium-238 decay chains being formed into a microbiological method of different isotopes of uranium, protactinium, thorium, actinium, radium, polonium and other elements (Figure 18).
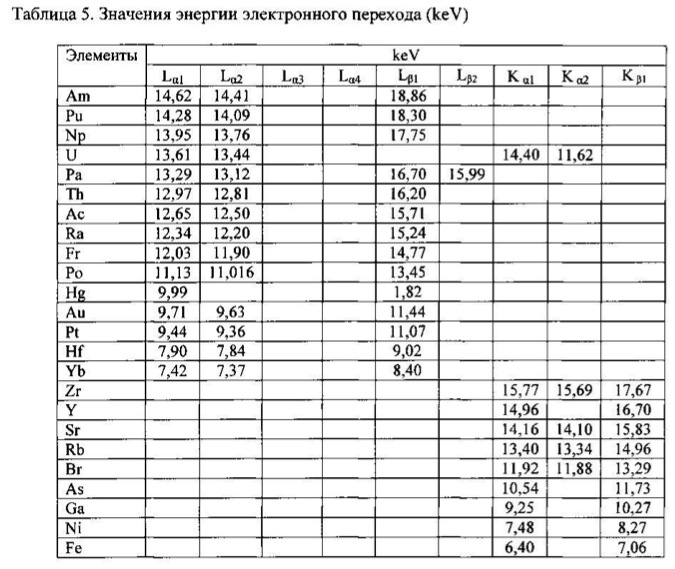
electronic transition energy (keV), which was determined by chemical elements by X-ray fluorescence (Figures 1 to 17) are shown in Table 5.
BIOTECHNOLOGICAL METHOD FOR ARTIFICIAL
PRODUCING OF ACTINIDES, OTHER VALUABLE RADIOACTIVE ELEMENTS,
THEIR ISOTOPES, AND STABLE ISOTOPES OF NOBLE METALS - PLATINUM
AND GOLD
V.M. Kurashov, T.V. Sakhno, R.G. Maksimov
[ PDF ]
V.M. Kurashov, T.V. Sakhno, R.G. Maksimov
[ PDF ]
Sulphide ores containing uranium-238 and thorium-230 are treated with water suspension of iron- and sulphur-oxidizing bacteria of Thiobacillus genus. Valuable radioactive elements and their isotopes such as polonium, francium, radium, actinium, protactinium, artificial isotopes of thorium and uranium, neptunium, americium, hafnium, ytterbium, as well as radioactive and stable isotopes of mercury and noble metals platinum and gold are artificially obtained. Transmutation of chemical elements and transformation of isotopes of chemical elements with the use of microorganisms are discovered and achieved.
ARTIFICIAL OBTAINING OF f-ELEMENTS –
ACTINIDES AND OTHER VALUABLE RADIOACTIVE ELEMENTS AND THEIR
ISOTOPES, AS WELL AS STABLE ISOTOPES OF PLATINUM AND GOLD WITH
THE USE OF MICROORGANISMS
V.M. Kurashov, T.V. Sakhno, R.G. Maksimov
V.M. Kurashov, T.V. Sakhno, R.G. Maksimov
Monazite (thorium-containing) sand of Indian Ocean Coast and uranium- and thorium-containing ore of Arabian Peninsula were treated separately with water suspension of Thiobacillus genera bacteria. Valuable radioactive elements and their isotopes such as hafnium, polonium, francium, radium, actinium, protactinium, artificial isotopes of thorium and uranium, neptunium, americium, as well as radioactive and stable isotopes of mercury and noble metals platinum and gold are artificially obtained. Transmutation of chemical elements and transformation of isotopes of chemical elements with the use of microorganisms are discovered and achieved. The invention also allows inactivating nuclear wastes by transfer hazardous for people radioactive isotopes into stable ones.
TRANSMUTATION OF CHEMICAL ELEMENTS AND
ISOTOPE TRANSFORMATION WITH THE USE OF BIOTECHNOLOGY
Kurashov V.M., Sakhno T.V.
[ PDF ]
Kurashov V.M., Sakhno T.V.
[ PDF ]
Radioactive raw material containing radioactive chemical elements or their isotopes are treated with water suspension of bacteria Thiobacillus genus. Radioactive wastes from nuclear fuel cycles are used as radioactive raw material. The method allows artificially obtaining polonium, francium, radium, actinium, protactinium, artificial isotopes of thorium and uranium. The invention allows obtaining valuable radioactive elements and their isotopes, as well as inactivating nuclear wastes with conversion of dangerous for people radioactive isotopes into stable ones.
Related :
RU2052223
METHOD FOR PRODUCING STABLE ISOTOPES DUE TO NUCLEAR TRANSMUTATION, SUCH AS LOW-TEMPERATURE NUCLEAR FUSION OF ELEMENTS IN MICROBIOLOGICAL CULTURES
METHOD FOR PRODUCING STABLE ISOTOPES DUE TO NUCLEAR TRANSMUTATION, SUCH AS LOW-TEMPERATURE NUCLEAR FUSION OF ELEMENTS IN MICROBIOLOGICAL CULTURES
Inventor(s): VYSOTSKIJ VLADIMIR; KORNILOVA ALLA; SAMOJLENKO IGOR
The invention relates to methods for producing stable isotopes and can be used in nuclear spectroscopy and applied nuclear physics technologies. A method of obtaining specific stable isotope by isolating them from natural source of multicomponent mixtures using isotope diffusion, mass spectrometry, or laser method (BM Andreev et al. The separation of stable isotopes of physical and chemical methods. M. 1982; Basov NG and etc. New methods of isotope separation. Advances of Physical Sciences, 1977, t.121, s.427).
The disadvantage of this method is the inability to obtain the necessary stable isotopes in their absence in the original multi-component environment. Furthermore, the method is very costly and time consuming.
A method of producing isotopes in the process of cold fusion, flowing at saturation palladium crystals or titanium with deuterium during electrolysis of heavy water (Browse: Tsarev VA Low-temperature nuclear fusion. Advances of Physical Sciences, 1992, t.160, s.19-20).
The method is based on the phenomenon of cold fusion which consists in the fact that the establishment of optimal conditions (temperature and structure of palladium or titanium matrix, the degree of saturation of the matrix with deuterium et al.) There is a fusion reaction D + D without imparting interacting deuterons high kinetic energy required hot reactions (thermonuclear) fusion to overcome the Coulomb barrier.
A method of obtaining stable isotopes by nuclear fusion of elements in microbial cultures, including the preparation of the nutrient medium for the growth of microbiological cultures deficient isotope resulting from transmutation, and contains the necessary source for transmutation isotopic components; cultivating in a nutrient medium microbiological cultures, these isotopes require for their growth; selection of the nutrient medium grown culture and selection of stable isotopes [2] In the known method describes the procedure for the cultivation of microbial cultures of Aspergillus niger IFO 4066, Penicillium chrysogenum IFO 4689; Phizopus nigricans IFO 5781; Mucor rouxii IFO 0369; Saccharomuces cerevisiae IFO 0308; Torulopsis utilis IFO 0396; Saccharomyces ellipideus IFO 0213; Hansenula anomala IFO 0118 in a nutrient media are aqueous solutions of a number of chemical compounds and deficient in one essential component for the growth of crops (potassium, magnesium, iron, calcium), and to control them in standard ENVIRONMENTS.
In the experiments of the method it showed that during the growth of the crops deficient in the corresponding element environments (there were no in these media specific data items) in the data elements obtained culture were present that can be connected only to their synthesis in nuclear transmutation of the other elements present, and isotopes. For example, magnesium formed by the reaction scheme Na23 + p1 & lowbar; & rarr; Mg24 A disadvantage of the known method is the low probability of transmutation nuclear reactions required due to unoptimized conditions of temperature and ionic-molecular composition of the nutrient medium, which is manifested in a small amount of atoms or ions.
Previous research on cold fusion shows that such reactions are successful only when a special selection of properties of the medium and temperature. Furthermore, the number of possible types of stable isotopes obtained in the known method and the corresponding one of the main elements that make up the grown microbial culture is insufficient. There are many types of isotopes, the receipt of which is of great interest, but which are not part of microbiological cultures.
The aim of the invention is to increase the rate of use of stable isotopes and increase the number of types of produced stable isotopes.
This is achieved by the fact that, in a process for the preparation of stable isotopes by nuclear transmutation type of cold fusion elements in microbial cultures, comprising the preparation of the nutrient medium for the growth of microbiological cultures deficient isotope resulting from transmutation, and contains necessary for the transmutation of the original isotopic components ; cultivating in a nutrient medium microbiological cultures, these isotopes require for their growth and development; isolation from the culture medium grown culture and isolation of stable isotopes in the culture medium is subjected to factors that increase in its concentration of free atoms or ions of hydrogen by breaking interatomic bonds.
In addition, the nutrient medium may be formed by heavy water D2O.
In addition, the composition of the nutrient medium necessary for transmutation include isotopic source components for which the result of the synthesis reactions are scarce nutrient medium for unstable isotopes, which are necessary for the formation and growth of microbial cultures, and are relative to parent subsidiary final stable isotopes.
As a factor degrading the interatomic bonds are used to supplement the nutrient medium or LiOD LiOH solution, and ionizing radiation.
The essence of the technical result of the invention is achieved as follows.
All the processes of nuclear transmutation on the basis of cold fusion (NTS) in biological cultures are at a very low (on the scale of conventional nuclear fusion, which requires temperatures of the order of many millions of degrees), the energy of the relative motion of interacting particles, which is certainly not enough to directly overcome the Coulomb reaction barrier . There are several different physical models describing the flow mechanism of the NTS. A prerequisite of the reaction is the formation of the NTS in the local environment of structural inhomogeneities within which the reactions take place and to form new isotopes. In the works (Vysotsky VI Kuzmin ON theory, mechanism and dynamics of barrier-free catalysis in solids. Preprint of the Institute of Theoretical Physics, Academy of Sciences of the USSR ITF-90-82R, Kiev, 1991; Vysotsky VI Kuzmin RN Mechanisms of barrier-free interaction with CNF nonequilibrium phenomena based on the Fermi condensate for numerically small ensemble and pulse dvuhdeytonnoy localization microcavities in optimal shape and size.
In: International Symposium on Cold fusion and new energy sources. Minsk, 1994, s.288-295) showed that most NTS phenomenon can effectively flow into the microcracks and microcavities with a characteristic size 2R1 & ap; 10-15 A or within the bulk inhomogeneities close to a parabolic potential profile at a ratio of the radius Ro and in the form of Uo Uo / R2o & ap; & Ap; 0,05-0,1 eV / A. STC process may take place not only in the interaction of light isotopes (for example, D + D, p + p), but involving the heavy isotope and an atom (or ion) hydrogen or deuterium D.
On the probability of the synthesis process is very strongly influenced by the ambient temperature and the atoms, as it affects the probability of settlement optimal for NTS energy levels in the microcavity, and residence time of the particles in a cavity: at high temperature has wasted particle quickly leaves the microcavity, and at low low probability falling particles in the microcavity, which already has another particle. If the microcavity are several particles, the temperature greatly affects their relative motion.
All prerequisites for the course of the NTS also occur during the growth of microbial cultures. In the area of growth because of the reproduction process, the formation and orientation of biomacromolecules is a rapid structural transformation of developing culture. Continuously there are structural microinhomogeneity with varying dimensions in time. When these dimensions for a certain time interval are close to optimal values characteristic or R1 Ro within microscopic inhomogeneities are prerequisites for the synthesis and transmutation.
This continuous process of structuring the inevitable passage of the characteristic dimensions of microscopic inhomogeneities R in terms of the optimal values of R1 or Ro at different times inevitably embraces all, without exception, the growing field of microbiological culture. This fact distinguishes from growing microcultures almost static palladium or titanium crystals (which are traditionally conducted experiments by NTS), in which the size, shape and number of microscopic inhomogeneities substantially fixed and there is no mechanism for bootstrapping NTS optimal conditions. If there are necessary for transmutation of initial isotopic components in a nutrient medium, as they enter the volume microheterogeneities with optimal parameters synthesis reaction occurs and there is an isotope that is initially absent from the culture medium (which was deficient in this isotope), but it is necessary to further culture growth. This isotope is immediately absorbed by microbiological culture and incorporated into its structure.
This process is repeated continuously throughout the growth area. After completion of the growth of the isotope obtained can be isolated from the resulting culture.
To be most effective this process requires that at least one of the initial isotopic component was in the form of free atoms or ions not bound in the molecule. Such a dissociation process can be random (fluctuating), but in doing so he will be characterized by a very low probability f & ap; exp (Ed / kT), where Ed dissociation energy, T the temperature.
In the present invention to provide such a requirement on the diet affect factors contributing to break atomic bonds and, as a consequence, an increase in the concentration of free atoms or hydrogen ions. In the case of the NTS in ordinary crystals performs this role addition of 0.1 mol / l KiOD in heavy water, in which the electrolysis is carried out with palladium or titanium electrodes in the proposed invention is also possible microbiological transmutation similar additive or LiOD LiOH solution in water culture medium solution. The use of other factors, such as weak ionizing radiation facilitating the formation of free radicals and H + H for the schemes: H2O + & planck; & omega; & Lowbar; & rarr; H2O ++ In addition, the possibility is greatly enhanced intended mode, i.e. It becomes possible to produce new types or the use of other isotopes of the starting components, if as a base for culture medium used instead of heavy water D2O ordinary (light) water H2O in the prior art.
Thus there is the possibility of nuclear transmutation reactions based on NTS involving deuterium D.
In addition to the direct transmutation isotopic source component. existing in the culture medium, lacking in the medium (deficient) stable isotope, which is required for the development of microbiological culture and therefore immediately absorbed by it, the method includes the step of obtaining from the original isotope component from initially deficient unstable isotopes, which are absorbed to the desired stable isotope. Thus it is possible to obtain such stable isotopes, which are not necessary for the growth of microbial cultures and are not included in their composition.
The invention is illustrated by the following specific examples of its implementation.
EXAMPLE 1. Preparing a nutrient medium containing saccharose (10%), ammonium tartrate (1%), Mg SO4 x 7 H2 O (0.25%), Ca HPO4 x2 x H2O (0.008%), K3PO4 (0,5%), Mn SO4 x 7 H2O (0,001%), water H2O (100%). The nutrient medium solution was added 0.1 mol / L LiOH to increase the concentration of free hydrogen atoms. After making a shaking cultivation conducted at 30 ° C for 24-72 h seed culture media of Saccharomyces yeast (Saccharomyces cerevisiae strain T-8). Growing cells were collected by tsentrifigurirovaniya. The precipitate was dried microbial mass disintegrated and stable isotope is determined by known physical and chemical methods.
EXAMPLE 2. Preparing a nutrient medium containing saccharose (10%), ammonium tartrate (1%), MgSO4 x 7 H2O (0,25%), Ca HPO4 x 2 H2O (0,008%), K3PO4 (0,5%), MnSO4 x 7 H2O (0,001%), water H2O (100%).
A solution of the nutrient medium is irradiated with ionizing radiation at a dose of less than 10 kGy, which simultaneously allows to achieve sterility of the environment. After making a seed culture medium-Saccharomyces yeast (Saccharomyces cerevisiae strain T-8) is carried out with shaking cultivation at 30 ° C for 72 to 24 hours. The grown cells were collected by tsentrifugurirovaniya. The precipitate was dried microbial mass disintegrated and stable isotope is determined by known physical and chemical methods.
EXAMPLE 3. Prepare medium deficient of potassium in the composition, sucrose 3; NaNO3 0,03; MgSO4 x 7 H2O 0,05; FeSO4 x 7 H2O 0,001; CaHPO4 0.008; Na2HPO4 0,1; NaCl 0,05; Water H2O to 100. Saturate the environment a major stable isotope Ar40 argon. The culture medium was added 0.1 mol / L LiOH to increase the concentration of free hydrogen atoms. Grown in this environment mold the culture, the prototype Mucor rontic.
During the synthesis reaction, Ar40 + p1 & lowbar; & rarr; k41 in the volume of developing microbial cultures formed a rare stable isotope K, which is absorbed by the mold and after its cultivation isolated by conventional chemical methods.
The grown cells were collected by centrifugation and the precipitate dried isotope obtained is isolated by known methods from the chemical residue.
EXAMPLE 4. Prepare medium deficient of potassium in the composition, sucrose 3; NaNO3 0,03; MgSO4 x 7 H2O 0,05; FeSO4x x 7 H2O 0,001; CaHPO4 0,008; Na2HPO4 0,1; NaCl 0,05; Water H2O to 100. Saturate the environment a major stable isotope Ar40 argon. The growth medium is irradiated with ionizing radiation at a dose of less than 10 kGy, which allows to simultaneously achieve sterility environment. Grown in this environment mold the culture, the prototype Mucor rontic.
During the synthesis reaction, Ar40 + p1 & lowbar; & rarr; k41 in the volume of developing microbial cultures formed a rare stable isotope K, which is absorbed by the mold and after its cultivation isolated by conventional chemical methods.
Growing cells were collected by centrifugation, the precipitate was dried, separated from the precipitate by known methods the resulting isotope.
EXAMPLE 5. Choosing Saccharomyces yeast culture from those for which growth requires manganese or nickel. Prepare a growth medium for these crops, which contains all the necessary for their growth chemical elements, as well as stable isotopes of Cr and Co, but does not contain manganese or nickel. During the cultivation of these crops with simultaneous exposure of one of the factors that increases the concentration of free atoms (as in Example 1), the reaction will occur Cr52 + p1 & lowbar; & rarr; Mn53 or Co58 + p1 & lowbar; & rarr; Ni60 Mn53 or products which are assimilated Ni60 growing culture. After completion of the cultivation cycle of the cultural synthesized or stable isotopes Ni60 Mn53 allocated chemical methods, the cultured cells are harvested, dried precipitate, the precipitate obtained is isolated from the isotope.
EXAMPLE 6. Form a medium deficient in iron (e.g., in composition, sucrose 3% NaNO3 0,3; K2HPO4 0,1; KCl 0,05; MgSO4 x 7 H2O 0,05; CaHPO4 0,008; MnSO4 x 7 H2O 0,001; heavy water D2O to 100). The nutrient medium solution was added 0.1 mol / L LiOH to increase the concentration of free hydrogen atoms. Is grown in this medium at T = 30C yeast culture, prototype Sccharomycrs cerevisae strain T-8, grown cells were collected by centrifugation, the precipitate was dried, and recovered the resulting isotope Fe57, known methods, formed during the reaction NTS Mn55 + d2 & lowbar; & rarr; Fe57 EXAMPLE 7 Example. Form a medium deficient in iron (e.g., in composition, sucrose 3; NaNO3 0,3; K2HPO4 0,1; KCL 0,05; MgSO4 x 7 H2O 0,05; CaHPO4 0,008; MnSO4 x 7 H2O 0,001; heavy water D2O to 100). The growth medium is irradiated with ionizing radiation at a dose of less than 10 kGy, which allows to simultaneously achieve sterility environment.
Is grown in this medium at T = 30C prototype culture Sccharomyces cerevisiae yeast strain T-8, grown cells were collected by centrifugation, the precipitate obtained is isolated and dried isotope Fe57, known methods, formed during the reaction NTS Mn55 + d2 & lowbar; & rarr; Fe57 EXAMPLE Example 8. A process for producing stable isotopes as a result of the collapse of the unstable parent isotope synthesized in the NTS in the deficit on the parent isotope medium involves growth in the microbiological culture in its composition.
Construct culture medium deficient in nitrogen composition, sucrose 3% K2HPO4 0,1; KCl 0,05; MgSO4 x 7 H2O 0,05; FeSO4 x 7 H2O 0,001; CaPHO4 0,008; MnSO4 x 7 H2O 0,001; Light water H2O to 100. The nutrient medium solution was added 0.1 mol / L LiOH to increase the concentration of free hydrogen atoms. Is grown in this medium at T = 30C Saccharomyces cerevisiae, strain T-8. During NTS reaction (with the participation of the main stable carbon isotope C12, which is a part of sucrose) C12 + p1 & lowbar; & rarr; N13 is formed unstable isotope N13, having a half-life of & ap; 10 min. This isotope immediately after the digest from the mold growing on nitrogen-deficient growth medium and fixed in the mold. Through time & tau; unstable isotope N13 spontaneously decays according to the scheme: N13 + & beta; + & lowbar; & rarr; C13 and converted into the final rare stable isotope C13, after which the whole mold growing stands in a known manner.
EXAMPLE 9. A process for producing stable isotopes as a result of the collapse of the unstable parent isotope synthesized in the NTS in the deficit on the parent isotope medium involves growth in the microbiological culture in its composition.
Construct culture medium deficient in nitrogen composition, sucrose 3; K2HPO4 0,1; KCl 0,05; MgSO4 x 7 H2O 0,05; FeSO4 x 7 H2O 0,001; CaHPO4 0,008; MnSO4x x 7 H2O 0,001, light water H2O to 100. The growth medium is irradiated with ionizing radiation at a dose of less than 10 kGy, which allows to simultaneously achieve sterility environment. Is grown in this medium at T = 30C Saccharomyces cerevisiae, strain T-8. During NTS reaction (with the participation of the main stable carbon isotope C12, included in the composition of sucrose) C12 + p1 & lowbar; & rarr; N13 is formed unstable isotope N13, having a half-life of & ap; 10 min. This isotope immediately after the digest from the mold growing on nitrogen-deficient growth medium and fixed in the mold. Through time & tau; unstable isotope N13 spontaneously decomposes under the scheme N13 & lowbar; & rarr; & Beta; ++ C13 is converted into a final m rare stable isotope C13, after which the whole mold growing stands in a known manner.
EXAMPLE 10. According to the scheme as in Example 8 and 9 may receive O17 isotope during the growth of microbial cultures require growth, respectively, in a nutrient medium fluoro deficient fluorine but containing stable isotope O16. The types of reactions that lead to the assimilation of the intermediate unstable isotopes produced during the STC, the following: O16 + p1 & lowbar; & rarr; F17 & lowbar; & rarr; & Beta; ++ O17 & tau; & Ap; 65 c After the cycle of growing culture and the collapse of the parent nuclei derived stable isotopes is isolated by conventional physical and chemical methods.
EXAMPLE 11. According to the scheme as in Example 8 and 9 may receive the isotope Si29 in the cultivation of microbial cultures require growth respectively, phosphorus in the nutrient medium deficient in phosphorus, but containing a stable isotope Si28. The type of reactions that lead to the assimilation of the intermediate unstable isotopes produced during the STC, the following: Si28 + p1 & lowbar; & rarr; p29 & lowbar; & rarr; & Beta; ++ Si29 & tau; & Ap; 4 c After the cycle of growing culture and the collapse of the parent nuclei derived stable isotopes is isolated by conventional physical and chemical methods.
EXAMPLE 12. According to the scheme as in Example 8 and 9 Fe57 isotope possible to obtain during the growth of microbial cultures require growth respectively, cobalt in a medium deficient in cobalt but containing stable isotope Fe56. The types of reactions that lead to the assimilation of the intermediate unstable isotopes produced during the STC, the following: Fe56 + p1 & lowbar; & rarr; Co57 & lowbar; & rarr; & Beta; ++ Fe57 & tau; & Ap; 271 days After completion of the cycle to the culture and the collapse of the parent nuclei The obtained stable isotopes is isolated by conventional physical and chemical methods.
http://web.mst.edu/~microbio/BIO221_2008/T_ferrooxidans.html
Thiobacillus ferrooxidans
Rachel Klapper
Rachel Klapper
The genus Thiobacillus, also known as Acidithiobacillus, contains colorless, rod-shaped bacteria. These bacteria have the ability to gain energy from the oxidation of reduced sulfur compounds. Therefore environmental requirements include the presence of reduced inorganic sulfur compounds. These bacteria are respiratory, preferentially utilizing oxygen as the terminal electron acceptor
Thiobacillus ferrooxidans is a gram negative, obligately autotrophic and aerobic Proteobacteria. These bacteria are motile, and possess polar flagella. T. ferrooxidans is an acidophile, living in environments with an optimal pH range of 1.5 to 2.5. T. ferrooxidans is also thermophilic, preferring temperatures of 45 to 50 degrees Celsius. The high temperature tolerance of the bacteria may be due in part to its high GC content of 55 to 65 mole percent.
T. ferrooxidans derives energy from oxidation of ferrous iron to ferric iron, and reduced-sulfur compounds to sulfuric acid. Fine sulfur deposits may accumulate in the cell wall of the bacteria. Other byproducts of metabolism (sulfuric acid) are sometimes associated with the oxidative corrosion of concrete and pipes. In soil environments, T. ferrooxidans is useful as a slow release source of phosphate and sulfate for soil fertilization.
T. ferrooxidans is the most active bacteria in mine wastes due to acid and metal pollution. Sites of extreme acid mine drainage also expose high levels of pyrite, an element that is readily oxidized by T. ferrooxidans. This pyrite-oxidizing capacity has been exploited in the industrial desulfurization of coal. T. ferrooxidans is used in industrial mineral processing and bioleaching processes. These bacteria have the ability to attack sulfide-containing minerals and convert insoluble sulfides of metals such as copper and zinc into their soluble metal sulfates. Metals recovered through this bioleaching process include copper, uranium and gold.
Sulfidic caves and areas of extreme acid mine drainage contain sites of pyrite deposits. In these areas extremely acidic (pH 0-1) microbial biofilms hang from the walls with a snot-like consistency. These colonies are known as snottites, and contain extremophilic bacteria. T. ferrooxidans and other members of the genus Thiobacillus (and/or similar bacteria) are thought to be a main component of the consortiums present in snottites. These bacteria derive energy from chemosynthesis of sulfur compounds and water which drain through the walls of the caves.
Hart, Steven. “Cave Slime.” NASA. 30 Mar. 2008. <http://www.nasa.gov/vision/universe/solarsystem/cave_slime.html>.
Kuenen, J. Gijs, et al. “The Genera Thiobacillus, Thiomicrospira, and Thiosphaera.” The Prokaryotes. Ed. Albert Balows, et al. New York: Springer-Verlog, 1992. 2638-9, 2650
Rawlings, Douglas, and Tomonobu Kusano. “Molecular Genetics of Thiobacillus ferroxidans.” Microbial Review 58.1 (1994): 39-55. 30 Mar. 2008. <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=372952>
“Thiobacillus-Microbewiki.” MicrobeWiki. 30 Mar. 2008. <http://microbewiki.kenyan.edu/index.php/Thiobacillus>.